一个虚拟筛选策略,重新利用抗叶酸化合物作为W. Bancrofti DHFR抑制剂。
IF 2.2
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
由班氏乌切里氏菌引起的淋巴丝虫病仍然是一项全球卫生挑战。bancrofti二氢叶酸还原酶(Wuchereria bancrofti dihydrofolate reductase, WbDHFR)是一种潜在的治疗靶点,因为DHFR在叶酸代谢和DNA合成中起着关键作用。在这项研究中,我们采用虚拟筛选工作流程来重新定位抗叶酸化合物作为WbDHFR抑制剂。利用蛋白质数据库的结构数据,我们构建了一个包含194种抗叶酸药物的文库,并将它们与WbDHFR叶酸结合位点对接。选择对接评分为-9 ~ -8 kcal/mol的化合物MTX、TSD001、TSD10和TSD25进行实验验证。TSD001 (Ki = 1 μM)对MTX具有纳米摩尔抑制活性,对MTX具有低微摩尔抑制活性。晶体学研究揭示了WbDHFR与甲氨蝶呤MTX(2.4 Å)、TSD001(2.9 Å)、TSD10(1.8 Å)和TSD25(2.1 Å)复合物的高分辨率结构,为结合相互作用提供了详细的见解。抑制剂常见的主要相互作用包括与Glu 32的氢键。这些发现突出了虚拟筛选工作流程的有效性,并为优化这些抗叶酸化合物抑制WbDHFR奠定了基础。该工作流程可应用于其他寄生虫DHFR酶,从而推进针对被忽视的热带病的药物发现工作。本文章由计算机程序翻译,如有差异,请以英文原文为准。
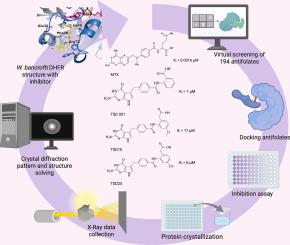
A virtual screening strategy to repurpose antifolate compounds as W. bancrofti DHFR inhibitors
Lymphatic filariasis, caused by Wuchereria bancrofti, remains a global health challenge. The enzyme Wuchereria bancrofti dihydrofolate reductase (WbDHFR) is a potential therapeutic target due to DHFR's critical role in folate metabolism and DNA synthesis. In this study, we employed a virtual screening workflow to repurpose antifolate compounds as WbDHFR inhibitors. Using structural data from the Protein Data Bank, we constructed a library of 194 antifolates and docked them to the WbDHFR folate binding site. Compounds methotrexate, TSD001, TSD10, and TSD25, with docking scores ranging from −9 to −8 kcal/mol, were selected for experimental validation. Inhibition assays demonstrated nanomolar activity with methotrexate and low micromolar activity with TSD001 (Ki = 1 μM). Crystallographic studies revealed high-resolution structures of WbDHFR in complex with methotrexate (2.4 Å), TSD001 (2.9 Å), TSD10 (1.8 Å) and TSD25 (2.1 Å), providing detailed insights into binding interactions. Major interactions common for the inhibitors include hydrogen bonds with Glu32. These findings highlight the effectiveness of the virtual screening workflow and establish a foundation for optimizing these antifolate compounds for WbDHFR inhibition. This workflow can be applied to other parasitic DHFR enzymes, advancing drug discovery efforts against neglected tropical diseases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.70
自引率
3.70%
发文量
463
审稿时长
27 days
期刊介绍:
Bioorganic & Medicinal Chemistry Letters presents preliminary experimental or theoretical research results of outstanding significance and timeliness on all aspects of science at the interface of chemistry and biology and on major advances in drug design and development. The journal publishes articles in the form of communications reporting experimental or theoretical results of special interest, and strives to provide maximum dissemination to a large, international audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


