在大型前瞻性队列中,生物年龄加速预测骨质疏松症和寿命缩短
IF 3.6
2区 医学
Q2 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
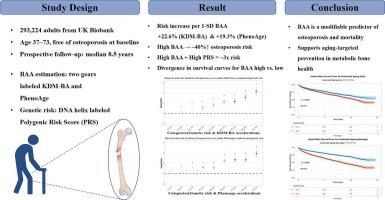
背景:骨质疏松症是一种主要的与年龄相关的肌肉骨骼疾病,但实足年龄并不能完全反映个体风险。生物年龄加速(BAA)作为全身性衰老的生物标志物,可能对骨质疏松症和寿命损失有较大的预测价值。方法:我们分析了来自英国生物银行(UK Biobank)的293,224名基线时无骨质疏松症的参与者的数据。BAA使用两种经过验证的模型- klemera - double Method Biological Age (KDM-BA)和PhenoAge进行估计。多基因风险评分(PRS)用于解释遗传易感性。多变量Cox模型检验了BAA和PRS与骨质疏松和全因死亡率的关系。结果在中位8.5年的随访中,9780名参与者出现了骨质疏松症。KDM-BA和PhenoAge加速每增加一个标准差(SD),骨质疏松症的风险分别增加22.6% (95% CI: 1.11, 1.36)和19.3% (95% CI: 1.12, 1.36)。与最低分位数的参与者相比,BAA最高分位数的参与者的风险增加了38 - 43%。同时具有高BAA和高PRS的个体,其骨质疏松的风险增加了近3倍,表明两者具有很强的叠加效应。加速衰老还与45岁时预期寿命减少1.3 - 1.8年有关,这与骨质疏松状况无关。结论生物加速衰老是骨质疏松和过早死亡的独立预测因子。将BAA纳入临床评估可以加强对高危个体的早期识别,并支持针对骨骼健康的老龄化干预措施。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Biological age acceleration predicts osteoporosis and reduced longevity in a large prospective cohort
Background
Osteoporosis is a major age-related musculoskeletal condition, yet chronological age does not fully capture individual risk. Biological age acceleration (BAA), as a biomarker of systemic aging, may offer greater predictive value for osteoporosis and lifespan loss.
Methods
We analyzed data from 293,224 participants in the UK Biobank cohort who were free of osteoporosis at baseline. BAA was estimated using two validated models—Klemera-Doubal Method Biological Age (KDM-BA) and PhenoAge. Polygenic risk scores (PRS) were used to account for genetic susceptibility. Multivariable Cox models examined associations of BAA and PRS with incident osteoporosis and all-cause mortality.
Results
Over a median follow-up of 8.5 years, 9780 participants developed osteoporosis. Each one standard deviation (SD) increase in KDM-BA and PhenoAge acceleration was associated with a 22.6 % (95 % CI: 1.11, 1.36) and 19.3 % (95 % CI: 1.12, 1.36) higher risk of osteoporosis, respectively. Participants in the highest tertile of BAA had a 38–43 % increased risk compared to those in the lowest tertile. Individuals with both high BAA and high PRS had nearly threefold higher osteoporosis risk, indicating a strong additive effect. Accelerated aging was also linked to a 1.3–1.8-year reduction in life expectancy at age 45, independent of osteoporosis status.
Conclusion
Accelerated biological aging is an independent predictor of osteoporosis and premature mortality. Integration of BAA into clinical assessment could enhance early identification of at-risk individuals and support aging-targeted interventions for skeletal health.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bone
医学-内分泌学与代谢
CiteScore
8.90
自引率
4.90%
发文量
264
审稿时长
30 days
期刊介绍:
BONE is an interdisciplinary forum for the rapid publication of original articles and reviews on basic, translational, and clinical aspects of bone and mineral metabolism. The Journal also encourages submissions related to interactions of bone with other organ systems, including cartilage, endocrine, muscle, fat, neural, vascular, gastrointestinal, hematopoietic, and immune systems. Particular attention is placed on the application of experimental studies to clinical practice.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


