揭示stat1激活巨噬细胞在腹主动脉瘤中的作用:来自scRNA-seq和预后特征发展的见解
IF 3.5
3区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
腹主动脉瘤(AAA)是一种危及生命的心血管疾病,没有有效的药物治疗。腹主动脉炎症和巨噬细胞浸润在AAA的发生、发展和破裂中起关键作用。然而,驱动这些过程的精确分子机制仍不清楚。为了解决这个问题,我们分析了来自三个AAA小鼠模型和两个健康对照的单细胞RNA测序数据集。我们的研究结果显示,AAA样本中巨噬细胞数量显著增加,与对照组中主要的M2表型相比,巨噬细胞向促炎M1极化转变。CellChat分析显示STAT1通路可能介导M1和M2巨噬细胞之间的细胞间通讯增强。SCENIC分析进一步发现STAT1在巨噬细胞中是一个中枢转录调节因子。我们还开发了一个预测AAA预后的十基因炎症特征,通过外部数据集进行验证,并通过Western blotting和实时PCR证实了STAT1的参与。这些发现强调了巨噬细胞极化和stat1驱动信号在AAA发病机制中的重要性。鉴定的基因标记为风险分层和治疗靶向提供了潜力,促进了对AAA的机制理解和临床管理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
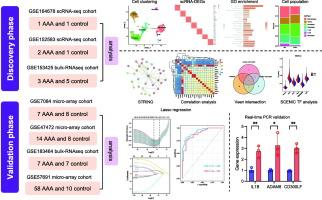
Unveiling the role of STAT1-activated macrophages in abdominal aortic aneurysm: Insights from scRNA-seq and prognostic signature development
Abdominal aortic aneurysm (AAA) is a life-threatening cardiovascular disorder with no effective drug treatment. Inflammation and macrophage infiltration in the abdominal aorta play a pivotal role in AAA development, progression, and rupture. However, the precise molecular mechanisms driving these processes remain unclear. To address this, we analyzed single-cell RNA sequencing datasets from three AAA mouse models and two healthy controls. Our findings revealed that AAA samples showed a marked increase in macrophage populations, with a shift toward pro-inflammatory M1 polarization compared to the predominantly M2 phenotype in controls. CellChat analysis revealed that the STAT1 pathway may mediate the enhanced intercellular communication between M1 and M2 macrophages. SCENIC analysis further identified STAT1 as a central transcriptional regulator in macrophages. We also developed a ten-gene inflammatory signature predictive of AAA prognosis, validated with external datasets, and confirmed STAT1's involvement via Western blotting and real-time PCR. These findings highlight the importance of macrophage polarization and STAT1-driven signaling in AAA pathogenesis. The identified gene signature offers potential for risk stratification and therapeutic targeting, advancing both mechanistic understanding and clinical management of AAA.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Vascular pharmacology
医学-药学
CiteScore
6.60
自引率
2.50%
发文量
153
审稿时长
31 days
期刊介绍:
Vascular Pharmacology publishes papers, which contains results of all aspects of biology and pharmacology of the vascular system.
Papers are encouraged in basic, translational and clinical aspects of Vascular Biology and Pharmacology, utilizing approaches ranging from molecular biology to integrative physiology. All papers are in English.
The Journal publishes review articles which include vascular aspects of thrombosis, inflammation, cell signalling, atherosclerosis, and lipid metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


