益生元改善亨廷顿病临床前模型的运动功能、认知和肠道健康。
IF 7.6
2区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
亨廷顿氏病(HD)是一种目前无法治愈的神经退行性疾病,其特征是精神、认知和运动缺陷,以及周围表现,包括胃肠道(GI)和免疫损伤。表达人类亨廷顿蛋白突变基因的R6/1小鼠HD模型具有良好的构建性和面部效度。据报道,临床和临床前HD患者存在肠道生态失调的证据,并与疾病症状密切相关,包括认知和行为结果。最近,高膳食纤维被证明可以通过未知的机制挽救HD小鼠的认知和情感缺陷并改善肠道功能。因此,我们旨在评估益生元调节肠道微生物在HD治疗中的治疗潜力。鉴于益生元如低聚果糖(FOS)和低聚半乳糖(GOS)作为有益微生物的底物的作用,我们假设长期补充FOS + GOS (PREB干预)可以改善与HD相关的肠道生态失调,从而减轻该临床前模型中的其他缺陷。在这里,R6/1 HD小鼠和野生型(WT)同窝对照随机分配,从6-20 周龄开始接受PREB或载药(饮用水)。我们评估了运动、认知和情感缺陷的发生和进展,以及GI参数和肠道宏观检查。此外,我们分析了第14周收集的粪便样本中的肠道微生物群(使用16S rRNA基因测序),并评估了它们的衍生短链脂肪酸(SCFAs)和支链脂肪酸(BCFAs)。与对照组相比,PREB改善了雌性HD小鼠的运动能力,增强了雌性HD和WT小鼠的认知能力。此外,PREB增加了HD和WT雄性小鼠的盲肠重量(两性)、粪便柔软度(雌性)和粪便中SCFAs的水平,包括丁酸盐(雄性)、醋酸盐(两性)和丙酸盐,但雌性HD小鼠仅如此。益生元干预还减少了女性晚发病时的肠道转运时间,HD和WT男性早发病时的粪便排出量,以及(女性)子宫旁脂肪。此外,在两性中,PREB降低了α-多样性,增加了β-多样性,包括在PREB处理的动物中产生scfa的微生物如动物双歧杆菌显著增加。综上所述,PREB有效地调节了HD小鼠的核心表型,特别是上述运动协调、认知和GI参数,并重塑了HD小鼠的肠道微生物群。这种益生元干预具有很强的安全性,可直接用于HD的未来临床试验。我们的研究结果表明,针对HD患者的肠道微生物群是一种合理的临床策略,并可能提供新的治疗方法,以延缓这种衰弱性疾病和其他具有类似表现的神经系统疾病的发病和/或进展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
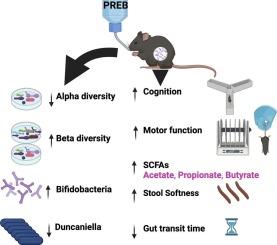
Prebiotics improve motor function, cognition and gut health in a preclinical model of Huntington’s disease
Huntington’s disease (HD) is a currently incurable neurodegenerative disorder characterised by psychiatric, cognitive and motor deficits, as well as peripheral manifestations, including gastrointestinal (GI) and immunological impairments. The R6/1 mouse model of HD, expressing a mutant human huntingtin transgene, exhibits excellent construct and face validity. Evidence of gut dysbiosis has been reported in clinical and preclinical HD and is strongly associated with disease symptoms, including cognitive and behavioural outcomes. Recently, high dietary fibre was shown to rescue cognitive and affective deficits and improve gut function in HD mice, by unknown mechanisms. Hence, we aimed to evaluate the therapeutic potential of gut microbial modulation by prebiotics in the treatment of HD. Given the well-documented role of prebiotics such as fructooligosaccharide (FOS) and galactooligosaccharide (GOS) as substrates of beneficial microbes, we hypothesised that chronic supplementation of FOS + GOS (PREB intervention) would ameliorate the gut dysbiosis associated with HD and consequently attenuate other deficits in this preclinical model.
Here, R6/1 HD mice and wild-type (WT) littermate controls were randomised to receive PREB or vehicle (drinking water) from 6-20 weeks of age. We assessed the onset and progression of motor, cognitive and affective deficits, as well as GI parameters and gut macroscopy. Additionally, we profiled the gut microbiota in faecal samples collected at week 14 (using 16S rRNA gene sequencing) and assessed their derivatised short-chain fatty acids (SCFAs) and branched-chain fatty acids (BCFAs).
Compared to vehicle controls, PREB improved the motor performance of female HD mice and enhanced the cognitive performance of female HD and WT mice. Furthermore, PREB increased caecal weight (both sexes), stool softness (females) and faecal levels of SCFAs, including butyrate (males), acetate (both sexes) and propionate in both HD and WT males but female HD mice only. The prebiotics intervention also decreased gut transit time in females at late onset and faecal output in both HD and WT males at early onset, as well as juxtauterine fat (in females). Furthermore, PREB decreased α-diversity and increased β-diversity in both sexes, including a remarkable increase in SCFA-producing microbes such as Bifidobacterium animalis in PREB-treated animals. Taken together, PREB effectively modulated the HD core phenotype, particularly motor coordination, cognition and GI parameters as described above, and remodelled the gut microbiota of HD mice.
This prebiotic intervention has a strong safety profile and is directly translatable to future clinical trials of HD. Our findings suggest that targeting the gut microbiota in HD is a plausible clinical strategy and may inform novel therapeutic approaches to delay the onset and/or progression of this debilitating condition and other neurological disorders with similar manifestations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
29.60
自引率
2.00%
发文量
290
审稿时长
28 days
期刊介绍:
Established in 1987, Brain, Behavior, and Immunity proudly serves as the official journal of the Psychoneuroimmunology Research Society (PNIRS). This pioneering journal is dedicated to publishing peer-reviewed basic, experimental, and clinical studies that explore the intricate interactions among behavioral, neural, endocrine, and immune systems in both humans and animals.
As an international and interdisciplinary platform, Brain, Behavior, and Immunity focuses on original research spanning neuroscience, immunology, integrative physiology, behavioral biology, psychiatry, psychology, and clinical medicine. The journal is inclusive of research conducted at various levels, including molecular, cellular, social, and whole organism perspectives. With a commitment to efficiency, the journal facilitates online submission and review, ensuring timely publication of experimental results. Manuscripts typically undergo peer review and are returned to authors within 30 days of submission. It's worth noting that Brain, Behavior, and Immunity, published eight times a year, does not impose submission fees or page charges, fostering an open and accessible platform for scientific discourse.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


