扩大赖氨酸乙酰化化学计量学的范围和临床影响
IF 2.8
2区 生物学
Q2 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
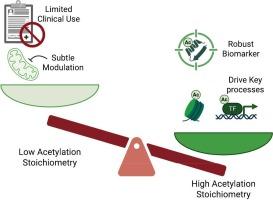
赖氨酸乙酰化,曾经主要被认为是一个组蛋白标记,现在被认为是一个广泛的蛋白质功能调节。最近在化学标记、同位素标记工作流程和数据独立采集质谱方面的突破使乙酰化化学计量学的精确、特定位点的量化成为可能。这种定量的“乙酰组学”方法揭示了一种“变阻器”模型,其中大多数乙酰化位点表现出低占用,作为微妙的调节剂,而高度乙酰化的赖氨酸子集(例如p53 c -端,AKT1,组蛋白)在基因表达,代谢和细胞命运中起关键的调节开关作用。位点特异性占用变化(如p53、PKM2)越来越多地作为癌症诊断、预后和治疗监测的强有力的生物标志物,通常超过mRNA或总蛋白水平。定量乙酰化数据现在指导靶向表观遗传治疗的发展,包括HDAC和p300/CBP抑制剂。除了肿瘤学,乙酰组学还可以精确定位心力衰竭的代谢瓶颈、神经退行性疾病的表观遗传缺陷和炎症信号节点。随着高通量工作流程、FFPE和液体活检相容性以及微流体平台的进步,乙酰化化学计量学已准备好用于临床翻译。我们强调了精准医学这一新兴维度的前景和挑战,强调需要整合多组学方法和强大的临床验证,以充分发挥定量乙酰组学在疾病诊断和治疗中的潜力。理解乙酰化在蛋白质中的占据程度,不仅仅是简单地确定乙酰化的存在与否,对生物学和医学具有深远的意义。这篇综述强调了乙酰化化学计量学的重要性,将先进的蛋白质组学技术与转化科学联系起来。我们强调,量化位点占用揭示哪些乙酰化事件真正调节酶的功能。例如,它可以识别哪些乙酰化事件真正调节酶活性或基因表达。此外,它还可以通过定性分析来突出癌症等疾病中不明显的分子变化。这些定量的见解为临床创新铺平了道路,包括基于乙酰化谱对患者进行分层的新型生物标志物和调节乙酰化水平的靶向治疗。总之,这项工作突出了过去二十年来蛋白质乙酰化研究的发展前景及其对翻译蛋白质组学的日益增长的影响,庆祝了全球研究界取得的里程碑。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Expanding the landscape of lysine acetylation stoichiometry and clinical impact
Lysine acetylation, once viewed primarily as a histone mark, is now recognized as a widespread regulator of protein function. Recent breakthroughs in chemical labeling, isotopic tagging workflows, and data-independent acquisition mass spectrometry enable precise, site-specific quantification of acetylation stoichiometry. This quantitative “acetylomics” approach reveals a “rheostat” model, where most acetylation sites exhibit low occupancy, acting as subtle modulators, while a subset of highly acetylated lysines (e.g., p53 C-terminus, AKT1, histones) serve as pivotal regulatory switches in gene expression, metabolism, and cell fate. Site-specific occupancy changes (e.g., p53, PKM2) increasingly serve as robust biomarkers for cancer diagnosis, prognosis, and therapeutic monitoring, often surpassing mRNA or total protein levels. Quantitative acetylation data now guide the development of targeted epigenetic therapies, including HDAC and p300/CBP inhibitors. Beyond oncology, acetylomics can pinpoint metabolic bottlenecks in heart failure, epigenetic deficits in neurodegenerative conditions, and inflammatory signaling nodes. With advances in high-throughput workflows, FFPE and liquid biopsy compatibility, and microfluidic platforms, acetylation stoichiometry is poised for clinical translation. We highlight both the promise and challenges of this emerging dimension of precision medicine, emphasizing the need for integrated multi-omics approaches and robust clinical validation to fully realize the potential of quantitative acetylomics in disease diagnosis and therapy.
Significance
Understanding the extent of acetylation occupancy in proteins, beyond simply determining presence or absence of acetylation, has profound implications for biology and medicine. This review emphasizes the importance of acetylation stoichiometry, connecting advanced proteomic technologies with translational science. We emphasize that quantifying site occupancy reveals which acetylation events truly modulate enzyme function. For instance, it can identify which acetylation events truly modulate enzyme activity or gene expression. Additionally, it can highlight molecular changes in diseases like cancer that are not apparent through qualitative analyses. These quantitative insights pave the way for clinical innovations, including novel biomarkers that stratify patients based on their acetylation profiles and targeted therapies that modulate acetylation levels. In summary, this work highlights the evolving landscape of protein acetylation research over the past two decades and its increasing influence on translational proteomics, celebrating milestones achieved by the global research community.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of proteomics
生物-生化研究方法
CiteScore
7.10
自引率
3.00%
发文量
227
审稿时长
73 days
期刊介绍:
Journal of Proteomics is aimed at protein scientists and analytical chemists in the field of proteomics, biomarker discovery, protein analytics, plant proteomics, microbial and animal proteomics, human studies, tissue imaging by mass spectrometry, non-conventional and non-model organism proteomics, and protein bioinformatics. The journal welcomes papers in new and upcoming areas such as metabolomics, genomics, systems biology, toxicogenomics, pharmacoproteomics.
Journal of Proteomics unifies both fundamental scientists and clinicians, and includes translational research. Suggestions for reviews, webinars and thematic issues are welcome.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


