Sprague-Dawley大鼠的三期VPA给药:一种具有成本效益的ASD模型,揭示了突触-线粒体-炎症轴作为治疗靶点
IF 5.1
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
摘要
为了克服传统单剂量丙戊酸(VPA)模型在自闭症谱系障碍(ASD)研究中的局限性,包括严重的母体毒性和不精确的胚胎暴露,本研究在Sprague-Dawley (SD)大鼠中采用三阶段序期VPA策略建立了一种具有成本效益的ASD模型。材料与方法妊娠SD大鼠于妊娠11.5、12.5、13.5天分别给予VPA(400→450→400 mg·kg−1)。评估了产妇/新生儿存活率、神经发育里程碑和行为表型(开放场地、三室社交能力、重复梳理)。分析大鼠前额皮质突触超微结构(透射电镜)、神经炎症(ELISA检测IL-1β、IL-6、TNF-α、IL-10)和氧化应激(CAT、SOD、GSH-Px、MDA)。优化后的方案消除了孕产妇死亡率(p <;0.01)和吸收(p <;0.0001),同时提高新生儿存活率(p <;0.01)和产仔数(12 ~ 16只)。模型大鼠表现出核心的ASD表型:社会缺陷(p <;0.0001),重复梳理(P <;0.01),神经发育迟缓。突触囊泡耗竭,线粒体嵴破坏,促炎细胞因子上调(p <;0.01),抗氧化抑制(p <;0.01)证实突触-线粒体-炎症轴失调。SD大鼠在表型保真度和建模效率方面优于C57BL/6小鼠。这项研究开创了一种平衡高ASD表型保真度和动物福利的三阶段VPA策略。突触-线粒体-炎症轴被认为是一个新的治疗靶点。SD大鼠为ASD机制和干预研究提供了一个优越、经济的平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。
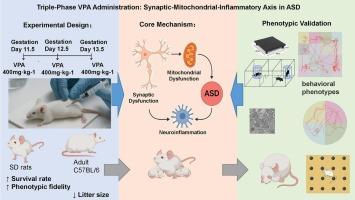
Triple-phase VPA administration in Sprague-Dawley rats: A cost-effective ASD model unveiling the synaptic-mitochondrial-inflammatory axis as a therapeutic target
Aims
To overcome limitations of traditional single-dose valproic acid (VPA) models in autism spectrum disorder (ASD) research—including severe maternal toxicity and imprecise embryonic exposure—this study established a cost-effective ASD model using a three-phase sequential VPA strategy in Sprague-Dawley (SD) rats.
Materials and methods
Pregnant SD rats received VPA (400 → 450 → 400 mg·kg−1) on gestational days 11.5, 12.5, and 13.5. Maternal/neonatal survival, neurodevelopmental milestones, and behavioral phenotypes (open field, three-chamber sociability, repetitive grooming) were assessed. Synaptic ultrastructure (transmission electron microscopy), neuroinflammation (ELISA for IL-1β, IL-6, TNF-α, IL-10), and oxidative stress (CAT, SOD, GSH-Px, MDA) in the prefrontal cortex were analyzed.
Key findings
The optimized protocol eliminated maternal mortality (p < 0.01) and resorption (p < 0.0001), while enhancing neonatal survival (p < 0.01) and litter size (12–16 pups). Model rats exhibited core ASD phenotypes: social deficits (p < 0.0001), repetitive grooming (P < 0.01), and delayed neurodevelopment. Synaptic vesicle depletion, mitochondrial cristae disruption, proinflammatory cytokine upregulation (p < 0.01), and antioxidant suppression (p < 0.01) confirmed synaptic-mitochondrial-inflammatory axis dysregulation. SD rats outperformed C57BL/6 mice in phenotypic fidelity and modeling efficiency.
Significance
This study pioneers a three-phase VPA strategy that balances high ASD phenotyping fidelity with animal welfare. The synaptic-mitochondrial-inflammatory axis is identified as a novel therapeutic target. SD rats provide a superior, cost-effective platform for ASD mechanism and intervention studies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Life sciences
医学-药学
CiteScore
12.20
自引率
1.60%
发文量
841
审稿时长
6 months
期刊介绍:
Life Sciences is an international journal publishing articles that emphasize the molecular, cellular, and functional basis of therapy. The journal emphasizes the understanding of mechanism that is relevant to all aspects of human disease and translation to patients. All articles are rigorously reviewed.
The Journal favors publication of full-length papers where modern scientific technologies are used to explain molecular, cellular and physiological mechanisms. Articles that merely report observations are rarely accepted. Recommendations from the Declaration of Helsinki or NIH guidelines for care and use of laboratory animals must be adhered to. Articles should be written at a level accessible to readers who are non-specialists in the topic of the article themselves, but who are interested in the research. The Journal welcomes reviews on topics of wide interest to investigators in the life sciences. We particularly encourage submission of brief, focused reviews containing high-quality artwork and require the use of mechanistic summary diagrams.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


