表达dnase1l3的树突状细胞通过降解中性粒细胞胞外陷阱促进CD8+ T细胞功能和抗pd -(L)1治疗效果
IF 44.5
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
摘要
CD8+ T细胞排斥和肿瘤微环境(TME)功能障碍是抗pd -(L)1治疗中最具挑战性的障碍之一。在这里,我们报道了肿瘤浸润性树突状细胞(DC)特异性表达的脱氧核糖核酸酶DNASE1L3与癌症患者抗pd -(L)1治疗的良好结果呈正相关。DNASE1L3在dc中的条件敲除通过损害CD8+ T细胞的浸润和效应功能,导致肿瘤生长增强,降低抗pd - l1的治疗效果。相反,注射DNASE1L3促进CD8+ T细胞浸润,减少TME衰竭,显著延缓肿瘤生长,增强抗pd - l1反应。DNASE1L3+ dc可以降解抑制肿瘤中CD8+ T细胞空间分布的中性粒细胞胞外陷阱,从而在人类癌症中建立细胞毒性CD8+ T细胞中心。我们的研究结果揭示了DC在调节肿瘤内CD8+ T细胞中的作用,并确定了DNASE1L3是改善抗pd -(L)1治疗的有希望的靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
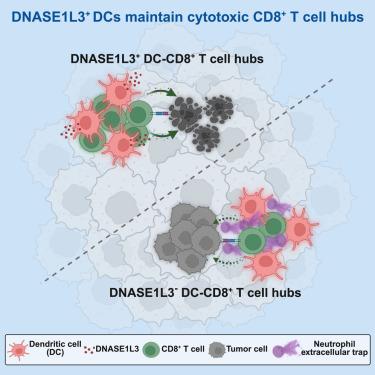
DNASE1L3-expressing dendritic cells promote CD8+ T cell function and anti-PD-(L)1 therapy efficacy by degrading neutrophil extracellular traps
CD8+ T cell exclusion and dysfunction in the tumor microenvironment (TME) are among the most challenging obstacles for anti-PD-(L)1 therapy. Here, we report that tumor-infiltrating dendritic cell (DC)-specific expression of the deoxyribonuclease, DNASE1L3, is positively correlated with favorable outcomes of anti-PD-(L)1 treatment in cancer patients. DNASE1L3 conditional knockout in DCs leads to enhanced tumor growth and diminishes anti-PD-L1 therapeutic efficacy by impairing infiltration and effector functions of CD8+ T cells. Conversely, injection with DNASE1L3 promotes CD8+ T cell infiltration and reduces exhaustion in the TME, significantly retarding tumor growth and enhancing anti-PD-L1 response. DNASE1L3+ DCs can degrade neutrophil extracellular traps that suppress the spatial distribution of CD8+ T cells in tumors, enabling establishment of cytotoxic CD8+ T cell hubs in human cancers. Our findings reveal a role of DC in regulating intratumoral CD8+ T cells and identify DNASE1L3 as a promising target to improve anti-PD-(L)1 therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer Cell
医学-肿瘤学
CiteScore
55.20
自引率
1.20%
发文量
179
审稿时长
4-8 weeks
期刊介绍:
Cancer Cell is a journal that focuses on promoting major advances in cancer research and oncology. The primary criteria for considering manuscripts are as follows:
Major advances: Manuscripts should provide significant advancements in answering important questions related to naturally occurring cancers.
Translational research: The journal welcomes translational research, which involves the application of basic scientific findings to human health and clinical practice.
Clinical investigations: Cancer Cell is interested in publishing clinical investigations that contribute to establishing new paradigms in the treatment, diagnosis, or prevention of cancers.
Insights into cancer biology: The journal values clinical investigations that provide important insights into cancer biology beyond what has been revealed by preclinical studies.
Mechanism-based proof-of-principle studies: Cancer Cell encourages the publication of mechanism-based proof-of-principle clinical studies, which demonstrate the feasibility of a specific therapeutic approach or diagnostic test.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


