卵巢癌中活化的MAFB通过WTAP竞争干扰m6A修饰,促进细胞骨架重塑和免疫微环境抑制。
IF 7.3
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
肿瘤微环境(TME)通过复杂的转录网络协调癌症的进展,但控制卵巢癌免疫逃避的分子机制仍然难以捉摸。在这里,通过整合多个临床队列和单细胞转录组学的免疫功能障碍特征,我们确定了MAFB是卵巢癌进展的主要调节因子。MAFB表达表现出分期依赖性升高,并与免疫检查点特征相关。在机制上,MAFB竞争性地与m6A甲基转移酶复合物的核心组分WTAP结合,从而拮抗靶基因mrna (WNT5A, CD55)的降解。这种非典型调控轴导致靶基因持续表达,通过细胞骨架蛋白重组、M2巨噬细胞极化和调节性T细胞浸润进一步协调肿瘤细胞侵袭和免疫景观重塑。患者队列的相关分析和临床前模型的治疗效果支持该途径的临床相关性。我们的研究结果揭示了MAFB通过细胞骨架重塑和免疫抑制促进卵巢癌进展的新机制,将转录调控与表转录组修饰联系起来,并确定了MAFB- wtap - cd55轴是卵巢癌的潜在治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
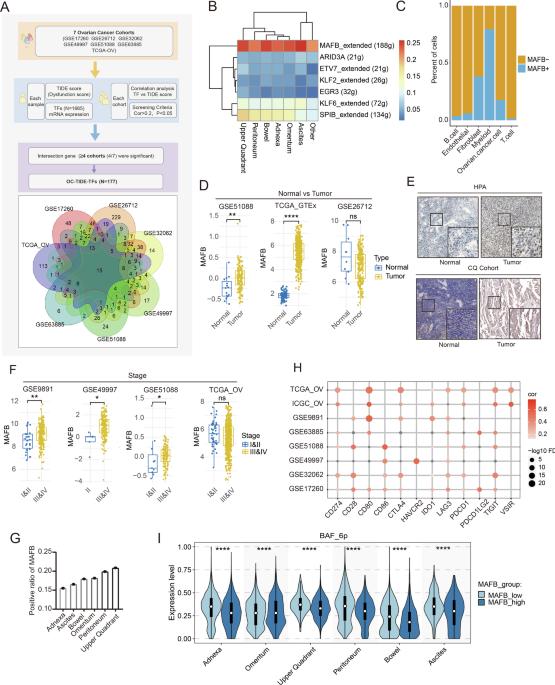
Activated MAFB in ovarian cancer promotes cytoskeletal remodeling and immune microenvironment suppression by interfering with m6A modifications through WTAP competition
The tumor microenvironment (TME) coordinates cancer progression through complex transcriptional networks, but the molecular mechanisms controlling immune evasion in ovarian cancer remain elusive. Here, by integrating immune dysfunction characteristics across multiple clinical cohorts and single-cell transcriptomics, we identified MAFB as a major regulator of ovarian cancer progression. MAFB expression exhibited stage-dependent elevation and was associated with immune checkpoint characteristics. Mechanistically, MAFB competitively binds to the core component WTAP of the m6A methyltransferase complex, thereby antagonizing the degradation of target gene mRNAs (WNT5A, CD55). This atypical regulatory axis leads to persistent expression of the target genes, further coordinating tumor cell invasiveness and immune landscape remodeling through cytoskeletal protein reorganization, M2 macrophage polarization, and regulatory T cell infiltration. Correlative analyses in patient cohorts and therapeutic effects in preclinical models support the clinical relevance of this pathway. Our findings uncover a novel mechanism by which MAFB promotes ovarian cancer progression through cytoskeletal remodeling and immune suppression, connecting transcriptional regulation with epitranscriptomic modifications, and identify the MAFB-WTAP-CD55 axis as a potential therapeutic target in ovarian cancer.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


