FXR调节肥胖患者肝脏线粒体功能以增加底物氧化
IF 14.6
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
摘要
代谢压力改变核受体的信号通路,包括胆汁酸受体FXR,它们对营养输入敏感。我们对肥胖或非肥胖患者的肝活检样本进行了以FXR chip -seq为中心的多组学分析,这些患者接受安慰剂或FXR激动剂奥贝胆酸治疗,以确定FXR信号通路的代谢适应。肥胖个体中FXR占据了更多的DNA结合位点,OCA激活FXR显著改变了转录输出。ChIP-seq和RNA-seq数据的整合表明,线粒体功能和底物氧化是肥胖个体中FXR激活选择性调节的主要代谢途径。FXR激活通过增强β-氧化和氧化磷酸化以及拮抗ROS的产生来恢复受损的底物氧化。与此相一致的是,肥胖患者在OCA治疗后还原型谷胱甘肽的含量恢复正常。综上所述,肥胖患者的FXR信号传导存在显著差异,包括DNA结合谱和转录程序的变化,这些变化增强了该患者群体对能量底物的利用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
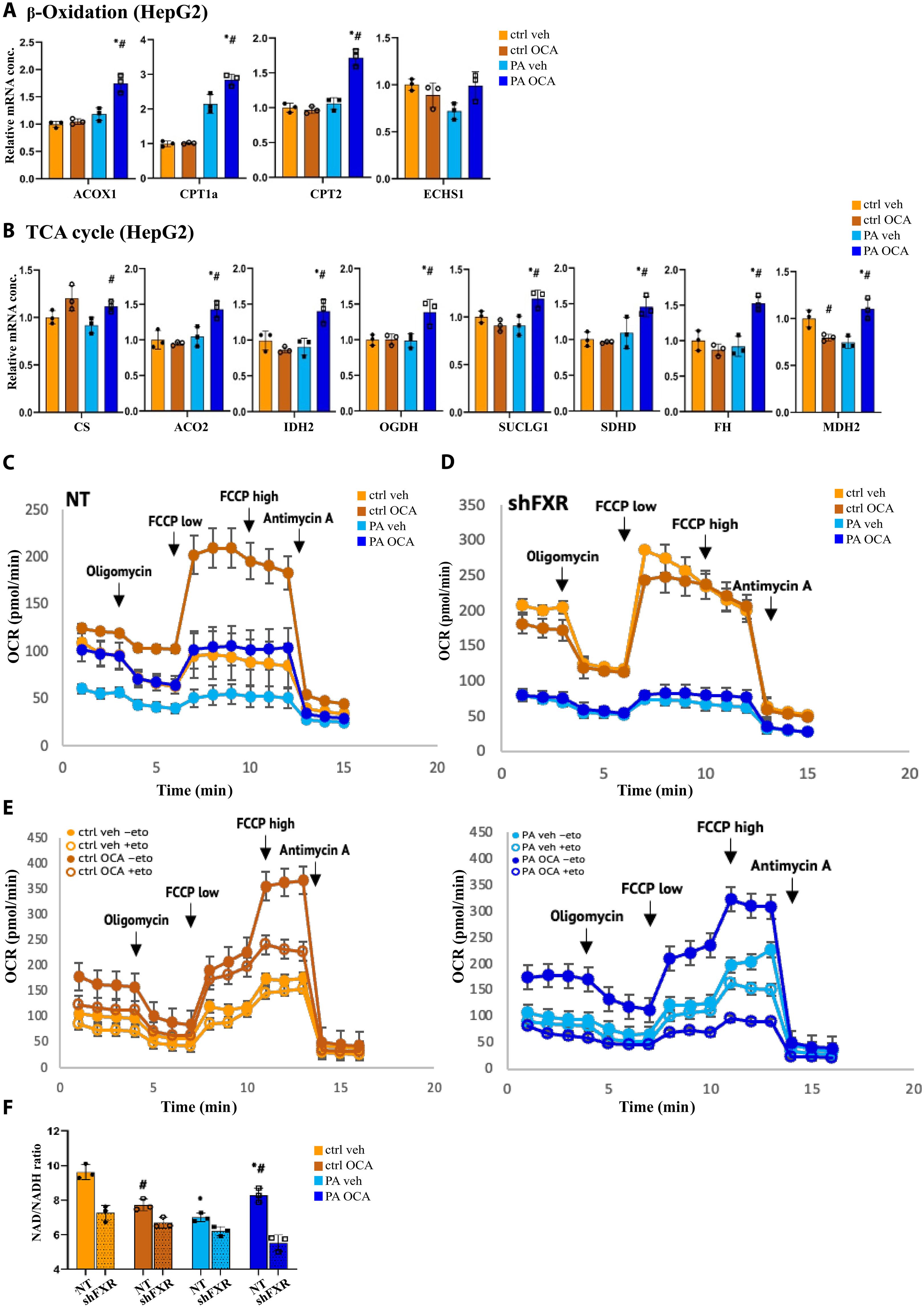
FXR adapts hepatic mitochondrial function to increased substrate oxidation in patients with obesity
Metabolic pressure shifts signaling pathways of nuclear receptors, including the bile acid receptor FXR, which are sensitive to nutritional inputs. We performed an FXR ChIP-seq–centered multiomic analysis of liver biopsy samples from individuals with or without obesity, who were treated with either placebo or the FXR agonist obeticholic acid, to define metabolic adaptions of FXR signaling pathways. FXR occupied substantially more DNA binding sites in individuals with obesity, and FXR activation by OCA robustly changed the transcriptional output. Integration of ChIP-seq and RNA-seq data showed that mitochondrial function and substrate oxidation were the top metabolic pathways selectively modulated by FXR activation in individuals with obesity. FXR activation restored compromised substrate oxidation by enhancing β-oxidation and oxidative phosphorylation along with antagonizing ROS production. In line with this, the amount of reduced glutathione in patients with obesity normalized after OCA treatment. In summary, FXR signaling profoundly differs in patients with obesity, consisting of changes in DNA binding profiles and transcriptional programs, which enhance energy substrate utilization in this patient cohort.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Translational Medicine
CELL BIOLOGY-MEDICINE, RESEARCH & EXPERIMENTAL
CiteScore
26.70
自引率
1.20%
发文量
309
审稿时长
1.7 months
期刊介绍:
Science Translational Medicine is an online journal that focuses on publishing research at the intersection of science, engineering, and medicine. The goal of the journal is to promote human health by providing a platform for researchers from various disciplines to communicate their latest advancements in biomedical, translational, and clinical research.
The journal aims to address the slow translation of scientific knowledge into effective treatments and health measures. It publishes articles that fill the knowledge gaps between preclinical research and medical applications, with a focus on accelerating the translation of knowledge into new ways of preventing, diagnosing, and treating human diseases.
The scope of Science Translational Medicine includes various areas such as cardiovascular disease, immunology/vaccines, metabolism/diabetes/obesity, neuroscience/neurology/psychiatry, cancer, infectious diseases, policy, behavior, bioengineering, chemical genomics/drug discovery, imaging, applied physical sciences, medical nanotechnology, drug delivery, biomarkers, gene therapy/regenerative medicine, toxicology and pharmacokinetics, data mining, cell culture, animal and human studies, medical informatics, and other interdisciplinary approaches to medicine.
The target audience of the journal includes researchers and management in academia, government, and the biotechnology and pharmaceutical industries. It is also relevant to physician scientists, regulators, policy makers, investors, business developers, and funding agencies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


