新型4-(2-甲氧基-5-((4-morpholinophenyl)亚氨基)甲基)苯氧基)功能化酞菁的合成、表征、电化学和电聚合性能
IF 2.1
3区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
合成了含4-(2-甲氧基-5-((4-morpholinophenyl)亚氨基)甲基)苯氧基)取代基的新型金属酞菁(6-8),并对其进行了表征。主要中间体和最终的酞菁配合物的结构通过FT-IR、1H NMR和质谱分析得到。采用循环伏安法(CV)和方波伏安法(SWV)研究了新合成的镍(II)(6)、钴(II)(7)和铜(II)(8)酞菁的电化学性能。其中,钴(II)酞菁(7)由于其中心金属离子的氧化还原活性,表现出明显的以金属为中心的氧化还原过程,而镍(II)(6)和铜(II)(8)类似物的电化学活性相对有限。这三种配合物都表现出几乎可逆的氧化还原行为,扫描速率相关的峰值电流变化证明了这一点。电聚合研究表明,聚合物膜厚度的增加导致电导率的降低和峰值电流的减弱,并伴随着氧化电位的变化。这些发现强调了中心金属离子和聚合物薄膜特性在调节电化学性能中的重要作用。合成的酞菁(6-8)也显示出修饰电极表面的良好潜力,提供改善的导电性,增强的稳定性,耐腐蚀性以及适用于传感器和保护涂层的应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
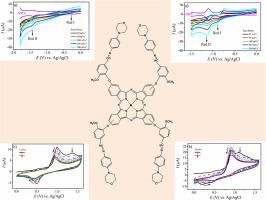
Synthesis, Characterization, Electrochemical and Electropolymerization Properties of Novel Phthalocyanines Functionalized with 4-(2-methoxy-5-(((4-morpholinophenyl)imino)methyl)phenoxy) Units
Novel metallophthalocyanines (6–8) bearing 4-(2-methoxy-5-(((4-morpholinophenyl)imino)methyl)phenoxy) substituents were synthesized and thoroughly characterized. The structures of the key intermediates and final phthalocyanine complexes were confirmed by FT-IR, 1H NMR, and mass spectrometry. The electrochemical properties of the newly synthesized nickel(II) (6), cobalt(II) (7), and copper(II) (8) phthalocyanines were investigated using cyclic voltammetry (CV) and square wave voltammetry (SWV). Among them, the cobalt(II) phthalocyanine (7) exhibited a distinct metal-centered redox process due to the redox-active nature of its central metal ion, while the nickel(II) (6) and copper(II) (8) analogs displayed relatively limited electrochemical activity. All three complexes showed nearly reversible redox behavior, as evidenced by scan rate-dependent peak current changes. Electropolymerization studies revealed that increasing polymer film thickness led to reduced conductivity and diminished peak currents, accompanied by shifts in oxidation potentials. These findings underscore the significant role of the central metal ion and polymer film characteristics in modulating electrochemical performance. The synthesized phthalocyanines (6–8) also demonstrated promising potential for modifying electrode surfaces, offering improved electrical conductivity, enhanced stability, corrosion resistance, and suitability for sensor and protective coating applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organometallic Chemistry
化学-无机化学与核化学
CiteScore
4.40
自引率
8.70%
发文量
221
审稿时长
36 days
期刊介绍:
The Journal of Organometallic Chemistry targets original papers dealing with theoretical aspects, structural chemistry, synthesis, physical and chemical properties (including reaction mechanisms), and practical applications of organometallic compounds.
Organometallic compounds are defined as compounds that contain metal - carbon bonds. The term metal includes all alkali and alkaline earth metals, all transition metals and the lanthanides and actinides in the Periodic Table. Metalloids including the elements in Group 13 and the heavier members of the Groups 14 - 16 are also included. The term chemistry includes syntheses, characterizations and reaction chemistry of all such compounds. Research reports based on use of organometallic complexes in bioorganometallic chemistry, medicine, material sciences, homogeneous catalysis and energy conversion are also welcome.
The scope of the journal has been enlarged to encompass important research on organometallic complexes in bioorganometallic chemistry and material sciences, and of heavier main group elements in organometallic chemistry. The journal also publishes review articles, short communications and notes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


