基于生物相容性的胆碱氨基酸离子液体氢键动力学及其温度依赖性
IF 3
4区 生物学
Q2 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
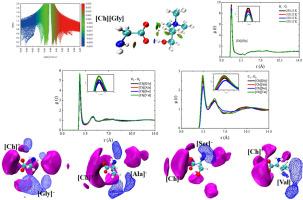
胆碱氨基酸离子液体([Ch][AA])是一种热稳定性高、生物降解性好、毒性低的绿色溶剂。本文采用密度泛函理论(DFT)计算和分子动力学(MD)模拟研究了四种[Ch][AA]的氢键动力学性质以及温度对它们的影响。通过原子-分子(AIM)和非共价相互作用(RDG)分析,发现阳离子的羟基氢(Hc1)原子和甲基氢(Hc2)原子分别与阴离子的羧酸氧(Oa)原子形成氢键(Oc-Hc1··Oa氢键、Cc-Hc2··Oa氢键)。Oc-Hc1··Oa氢键相互作用强于Cc-Hc2··Oa氢键相互作用。同时,径向分布函数(rdf)分析进一步证实Oc-Hc1···Oa氢键相互作用更强。随着阴离子链长的增加,Oc-Hc1··Oa和Cc-Hc2··Oa氢键相互作用的强度增大。随着温度的升高,粒子间的相互作用减弱,扩散系数和离子电导率增大。盒子快照和空间分布函数(sdf)分析表明,[Ch][AA]中阴离子之间发生聚集现象,形成簇簇,这主要是由于阴离子之间的侧链相互作用所致。随着阴离子链长的增加,这种聚集现象更加明显。此外,还发现羟基可以参与形成更多的氢键网络,从而增强离子液体之间的分子间作用力,使体系更具粘性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Hydrogen bond kinetics and their temperature dependence of biocompatibility-based choline amino acid ionic liquids
Choline amino acid ionic liquids ([Ch][AA]) are green solvents with high thermal stability, good biodegradability and low toxicity. In this work, the hydrogen bond kinetic properties of the four [Ch][AA] and the effect of temperature on them were investigated using density functional theory (DFT) calculations and molecular dynamics (MD) simulations. By atom-in-molecule (AIM) and noncovalent interaction (RDG) analysis, it was found that the hydroxyl hydrogen (Hc1) atom and the methyl hydrogen (Hc2) atom of the cation form hydrogen bonds with the carboxylate oxygen (Oa) atom of the anion (Oc-Hc1···Oa hydrogen bond, Cc-Hc2···Oa hydrogen bond), respectively. However, the Oc-Hc1···Oa hydrogen bond interaction was stronger than the Cc-Hc2···Oa hydrogen bond interaction. Simultaneously, radial distribution functions (RDFs) analysis further confirmed that the Oc-Hc1···Oa hydrogen bond interaction was stronger. As chain length of anion increases, the strength of the Oc-Hc1···Oa and Cc-Hc2···Oa hydrogen bond interactions increase. Furthermore, with the increase of temperature, the interactions between the particles weaken, while the diffusion coefficient and the ionic conductivity increase. Box snapshots and Spatial distribution functions (SDFs) analysis indicated that aggregation phenomenon occurs between anions in [Ch][AA], forming clusters, which was mainly due to the side chain interaction between anions. As chain length of anion increases, this aggregation phenomenon became more evident. In addition, it was found that hydroxyl groups can participate in the formation of more hydrogen bonding networks, which enhances the intermolecular forces between the ionic liquids and makes the system more viscous.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of molecular graphics & modelling
生物-计算机:跨学科应用
CiteScore
5.50
自引率
6.90%
发文量
216
审稿时长
35 days
期刊介绍:
The Journal of Molecular Graphics and Modelling is devoted to the publication of papers on the uses of computers in theoretical investigations of molecular structure, function, interaction, and design. The scope of the journal includes all aspects of molecular modeling and computational chemistry, including, for instance, the study of molecular shape and properties, molecular simulations, protein and polymer engineering, drug design, materials design, structure-activity and structure-property relationships, database mining, and compound library design.
As a primary research journal, JMGM seeks to bring new knowledge to the attention of our readers. As such, submissions to the journal need to not only report results, but must draw conclusions and explore implications of the work presented. Authors are strongly encouraged to bear this in mind when preparing manuscripts. Routine applications of standard modelling approaches, providing only very limited new scientific insight, will not meet our criteria for publication. Reproducibility of reported calculations is an important issue. Wherever possible, we urge authors to enhance their papers with Supplementary Data, for example, in QSAR studies machine-readable versions of molecular datasets or in the development of new force-field parameters versions of the topology and force field parameter files. Routine applications of existing methods that do not lead to genuinely new insight will not be considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


