一个单氢键保证白喉链杆菌的铜原血红素脱羧酶的构象稳定性和活性
IF 3.2
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
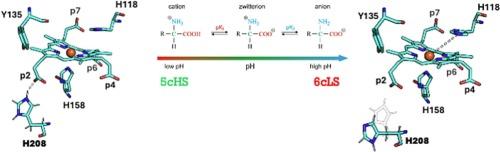
酶的活性位点结构是由底物和氨基酸残基之间的许多相互作用决定的,并针对特定和有效的底物周转进行了优化。在铜原血红素脱羧酶(ChdC)的情况下,活性位点的结构很好地描述了结构和热力学手段。铜原卟啉脱羧酶通过氧化将两个丙酸基团脱羧为乙烯基,将铁的铜原卟啉III(铜原卟啉)转化为铁原卟啉IX(血红素b)。在这项研究中,我们研究了一个位于2 (p2)位置丙酸附近的精氨酸残基(R208, ChdC),该残基已被证明在活性位点结构中具有重要的空间位作用。本文通过研究白喉链杆菌中coproheme脱羧酶的几个R208变体,重点研究了其立体作用的分子基础和催化作用。分析R208转化为His、Lys、Glu、Asp和Ser(丝氨酸模拟厚壁菌门中chdc的情况)有助于加深我们对该酶及其反应机制的理解。通过实验生化研究和分子动力学模拟,我们确定了一个特别重要的单氢键,证明了质子化状态的重要性,并且R208是催化转换过程中没有直接机制作用的基本残留物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
One single hydrogen bond guarantees conformational stability and activity in coproheme decarboxylase from Corynebacterium diphtheriae
Active site architectures of enzymes are defined by many interactions between substrate and amino acid residues and are optimized for specific and efficient substrate turnover. In the case of coproheme decarboxylase (ChdC) the active site architecture is well described by structural and thermodynamic means. Coproheme decarboxylases transform iron coproporphyrin III (coproheme) into iron protoporphyrin IX (heme b) by oxidatively decarboxylating two propionate groups to vinyls. In this study we have investigated an arginine residue (R208, ChdC from Corynebacterium diphtheriae) in close proximity to propionate at position 2 (p2) that has been indicated to have an important steric role within the active site architecture. Here we focus on the molecular basis of its steric role and the catalytic consequences by investigating several R208 variants of coproheme decarboxylase from the Actinobacterium Corynebacterium diphtheriae. Analyses of the exchange of R208 into His, Lys, Glu, Asp, and Ser (serine mimics the situation of ChdCs in Firmicutes) help to deepen our understanding of this enzyme and its reaction mechanism. By employing experimental biochemical studies and molecular dynamics simulations we identify one single hydrogen bond of particular importance, proving that the protonation state matters and that R208 is an essential residue without having a direct mechanistic role during catalytic turnover.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


