外源性tau聚集物的内化与早期内吞标记物和溶酶体共定位
IF 2.2
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
Tau蛋白的扩散和内化与阿尔茨海默病和Tau病变的疾病进展有关。Tau内化在传播中起着关键作用。参与脑监测和清除聚集物的细胞包括实质边界巨噬细胞(小胶质细胞)、血管周围巨噬细胞、脑膜和脉络膜丛巨噬细胞。然而,在中风等事件中,tau淀粉样蛋白会破坏血脑屏障(BBB)。促进汇总资料的传播。因此,我们评估了tau聚集体是否对半透层(如DOPC)有影响。纳米压痕显示全长tau单体和tau 4R(微管结合域)与DOPC的相互作用不同,淀粉样蛋白增加Fp(临界力),但不破坏DOPC。然后,为了分析聚集物对N2a细胞的影响,我们将tau聚集物孵育24 h;导致轴突样结构减少,损害细胞完整性。随后,用264.7细胞(小鼠巨噬细胞)培养的tau聚集体显示,4R微管结合域与Rab5和Lamp1共定位,这表明溶酶体在tau聚集体的清除中起关键作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
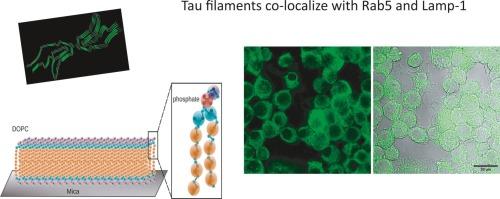
Internalization of external tau aggregates co-localize with early endocytic markers and lysosomes
Tau's spread and internalization are related to disease progression in Alzheimer's disease and tauopathies. Tau internalization plays a critical role in the spreading. The cells involved in brain surveillance involved in the clearance of aggregates include parenchymal-border macrophages (microglia), perivascular macrophages, and meningeal and choroid plexus macrophages. However, in events such as strokes, or tau amyloids can breach the blood-brain barrier (BBB). Facilitating the dissemination of aggregates. Thus, we evaluated whether the tau aggregates have effect over a semi-permeable layer such as DOPC. Nano-indentation showed that tau monomers of full length and tau 4R (microtubule binding domain) interact differently with DOPC, and the amyloids increase the Fp (critical force) but not disrupt the DOPC. Then, to analyze the effect of aggregates on N2a cells, we incubated tau aggregates for 24 h; resulting in the decrease of axon-like structures compromising the cell integrity. Afterwards, cultured tau aggregates with raw 264.7 cells (mouse macrophages) showed that the 4R microtubule-binding domain co-localize with Rab5 and Lamp1, suggesting a key role to lysosomes in the clearance of tau aggregates.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biophysical chemistry
生物-生化与分子生物学
CiteScore
6.10
自引率
10.50%
发文量
121
审稿时长
20 days
期刊介绍:
Biophysical Chemistry publishes original work and reviews in the areas of chemistry and physics directly impacting biological phenomena. Quantitative analysis of the properties of biological macromolecules, biologically active molecules, macromolecular assemblies and cell components in terms of kinetics, thermodynamics, spatio-temporal organization, NMR and X-ray structural biology, as well as single-molecule detection represent a major focus of the journal. Theoretical and computational treatments of biomacromolecular systems, macromolecular interactions, regulatory control and systems biology are also of interest to the journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


