c环硝基取代脱氢枞胺类似物的合成和生物学评价:体外细胞毒性、DNA亲和力和分子对接研究
IF 3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
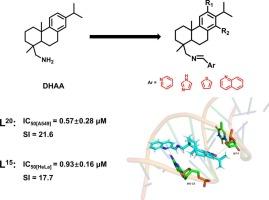
为了追求更有效的抗癌治疗,我们开发了一系列创新的脱氢二乙胺(DHAA)衍生物,特别强调在C-18希夫碱杂环结构的基础上对c环进行硝基修饰。我们在体外评估了这些化合物对人类肿瘤细胞系MCF-7(乳腺癌)、A549(肺癌)、HeLa(宫颈癌)和HepG2(肝癌)以及非恶性细胞系HUVEC(人脐静脉内皮细胞)的细胞毒性作用。DHAA衍生物L15和L20分别对HeLa和A549细胞具有较高的细胞毒活性,而对非恶性HUVEC细胞具有较低的细胞毒活性。同时对DHAA衍生物的细胞毒性作用机制进行了初步探讨。提示诱导细胞凋亡可能是其主要机制。利用吸收光谱分析和溴化乙啶(EB)荧光位移实验研究了DHAA衍生物与HS-DNA的相互作用,结果表明DHAA衍生物与DNA的结合是以插层方式进行的。分子对接研究表明,在碳-氢相互作用和π-供体氢键的促进下,DHAA衍生物与DNA具有较强的结合亲和力。构效关系的讨论表明,硝基尤其是14-硝基的引入显著提高了DHAA的细胞毒性。化合物L15和L20具有显著的细胞毒性和高选择性,是有潜力的抗肿瘤药物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Synthesis and biological evaluation of C-ring nitro-substituted dehydroabietylamine analogs: in vitro cytotoxicity, DNA affinity, and molecular docking studies
In pursuit of more effective anticancer treatments, we developed a series of innovative dehydroabietylamine (DHAA) derivatives, with particular emphasis on introducing nitro modifications to the C-ring based on incorporating C-18 Schiff base heterocyclic structures. We evaluated the cytotoxic effects of these compounds in vitro against a panel of human tumor cell lines – MCF-7 (breast cancer), A549 (lung cancer), HeLa (cervical cancer), and HepG2 (liver cancer) - as well as the non-malignant cell line HUVEC (human umbilical vein endothelial cells). DHAA derivatives L15 and L20, with C-ring 14-nitro substitution based on C-18 Schiff base heterocyclic modification, showed higher cytotoxic activity against HeLa and A549 cells, respectively, while demonstrating significantly lower cytotoxicity to non-malignant HUVEC cells. Meantime the mechanism of cytotoxicity of DHAA derivatives was preliminarily investigated. The result suggested that inducing cell apoptosis might be the primary mechanism. The interaction between DHAA derivatives and HS-DNA was studied using absorption spectral analysis and ethidium bromide (EB) fluorescence displacement experiments, the results exhibited that the binding of DHAA derivatives to DNA was in the intercalative mode. The molecular docking study demonstrated that DHAA derivatives exhibited a strong binding affinity to DNA, facilitated by carbon‑hydrogen interactions and π-donor hydrogen bonds. The structure-activity relationship discussion implied that introduction of the nitro-group, especially the 14-nitro group, significantly improved the cytotoxicity of DHAA. The significant cytotoxicity and high selectivity exhibited by compounds L15 and L20 suggested their potential as promising antitumor medicines.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


