新型COX-2特异性抑制剂的虚拟筛选、合成、优化及抗炎活性研究
IF 3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
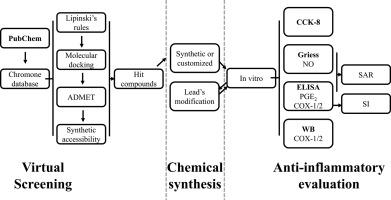
在本研究中,我们率先使用SMARTS筛选构建了染色体数据库,并利用Discovery Studio通过LibDock和CDOCKER实现COX-2的精确分子对接,成功锁定了海量染色体库中的7个命中化合物。在细胞实验中,Q7对lps诱导的RAW264.7细胞PGE2 (IC50 = 68.23±8.94 μM)和NO (IC50 = 44.83±2.01 μM)均有显著抑制作用,其抗炎活性优于传统抗炎药布洛芬[IC50(PGE2) = 246.5±3.8 μM]和L-canavanine [IC50(NO) = 440.0±7.9 μM]。但其活性仍不如塞来昔布[IC50 (PGE2) = 0.882±0.021 μM], Q7的western blot进一步证实其对COX-2的靶向抑制作用。在此基础上,通过柔性对接设计对Q7的结构进行优化,依次合成了30个具有一定抗炎活性的Q7衍生物,其中Q7 - 9 [IC50(PGE2) = 0.209±0.022 μM, IC50(COX-2) = 0.121±0.010 μM], Q7 - 25 [IC50(PGE2) = 0.267±0.017 μM, IC50(COX-2) = 0.228±0.021 μM]和Q7 - 26 [IC50(PGE2) = 0.161±0.018 μM,IC50(COX-2) = 0.137±0.004 μM]抗炎活性优于塞来昔布,选择性指数中等(SIQ7-9 >;826)。Q7-28 (IC50 = 0.014±0.001μM)和Q7-29 (IC50 & lt;0.0128 μM)对NO有显著抑制作用。此外,通过构建3D-QSAR模型,系统揭示了色素和异黄酮分子对COX-2和iNOS靶点的抗炎结构。本研究为新的色素非甾体类抗炎药的研发提供了重要的参考和依据,Q7-9有望成为具有高安全性和特异性COX-2抑制剂的候选药物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Virtual screening, synthesis, optimization and anti-inflammatory activity of novel chromones as specific COX-2 inhibitors
In this study, we pioneered the use of SMARTS screening to construct a chromone database, and the Discovery Studio was used to achieve precise molecular docking of COX-2 through LibDock and CDOCKER, and successfully locked 7 hit compounds from the massive chromone library. In cell experiments, Q7 had a significant inhibitory effect on LPS-induced PGE2 (IC50 = 68.23 ± 8.94 μM) and NO (IC50 = 44.83 ± 2.01 μM) in RAW264.7 cells, and its anti-inflammatory activity was superior to that of the traditional anti-inflammatory agents ibuprofen [IC50(PGE2) = 246.5 ± 3.8 μM] and L-canavanine [IC50(NO) = 440.0 ± 7.9 μM]. Still, the activity was not as good as that of celecoxib [IC50 (PGE2) = 0.882 ± 0.021 μM], and the western blot of Q7 further confirmed its targeted inhibition of COX-2. On this basis, the structure of Q7 was optimized by flexible docking design, and 30 Q7 derivatives were synthesized one by one, all of which showed certain anti-inflammatory activities, among which Q7–9 [IC50(PGE2) = 0.209 ± 0.022 μM, IC50(COX-2) = 0.121 ± 0.010 μM], Q7–25 [IC50(PGE2) = 0.267 ± 0.017 μM, IC50(COX-2) = 0.228 ± 0.021 μM] and Q7–26 [IC50(PGE2) = 0.161 ± 0.018 μM, IC50(COX-2) = 0.137 ± 0.004 μM] exhibited anti-inflammatory activity better than celecoxib and had a moderate selectivity index (SIQ7–9 > 826). Q7–28 (IC50 = 0.014 ± 0.001 μM) and Q7–29 (IC50 < 0.0128 μM) had significant inhibitory effects on NO. In addition, by constructing a 3D-QSAR model, the anti-inflammatory structures of chromone and isoflavone molecules for COX-2 and iNOS targets were systematically revealed. This study provided an important reference and basis for the research and development of new chromone non-steroidal anti-inflammatory drugs, and Q7–9 was expected to be a candidate drug with high safety and specific COX-2 inhibitors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


