抑制和激活KIRs识别RIFINs: NK细胞控制疟疾的双刃剑机制
IF 52.7
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
Sakoguchi等人在《自然》杂志最近发表的一篇文章中揭示了病原体衍生配体与激活人类自然杀伤(NK)细胞受体之间长期寻找的缺失联系该研究鉴定了恶性疟原虫(P. falciparum)重复穿插家族(RIFIN)蛋白的一个进化支,它不仅结合抑制KIR2DL1受体,而且引人注意的是,还参与激活KIR2DS1受体,从而为疟疾中的NK细胞调节提供了新的见解,并扩大了我们对先天免疫反应中宿主-病原体相互作用的理解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
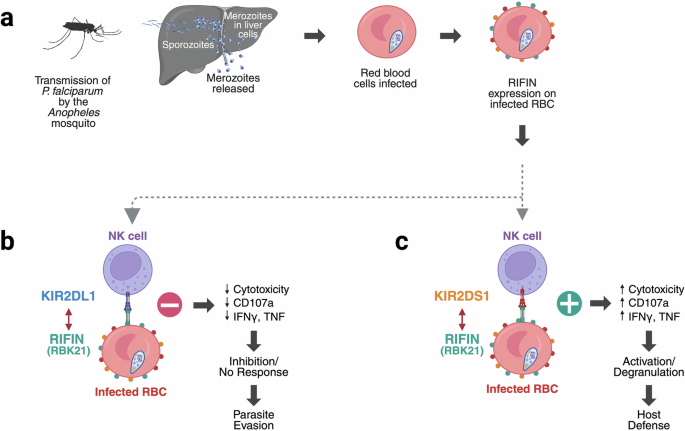
Both inhibitory and activating KIRs recognize RIFINs: a dual-edged mechanism of NK cell control in malaria
In a recent publication in Nature, Sakoguchi et al. reveal a long-sought missing link between pathogen-derived ligands and activating human natural killer (NK) cell receptors.1 The study identifies a clade of Plasmodium falciparum (P. falciparum) repetitive interspersed family (RIFIN) proteins that not only bind to the inhibitory KIR2DL1 receptor but, strikingly, also engage the activating KIR2DS1 receptor, thereby offering new insight into NK cell regulation in malaria and expanding our understanding of host–pathogen interaction in innate immune responses.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Signal Transduction and Targeted Therapy
Biochemistry, Genetics and Molecular Biology-Genetics
CiteScore
44.50
自引率
1.50%
发文量
384
审稿时长
5 weeks
期刊介绍:
Signal Transduction and Targeted Therapy is an open access journal that focuses on timely publication of cutting-edge discoveries and advancements in basic science and clinical research related to signal transduction and targeted therapy.
Scope: The journal covers research on major human diseases, including, but not limited to:
Cancer,Cardiovascular diseases,Autoimmune diseases,Nervous system diseases.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


