cocf3系结烯二酮和吡唑啉酮的对映选择性葡萄系Michael有机级联:获得螺旋融合的线性氧-三醌。
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们报道了一种流线型的螺旋融合多环支架的有机催化对映选择性构建,获得了优异的产率和非对映性和对映选择性。双功能方酰胺催化剂可实现不对称VMA反应,随后是乙烯基吡唑酮与三氟乙酰基系结烯二酮的质子转移/醛醇/乙酰化级联反应。此外,标题产品的可伸缩性和合成后转换突出了它们的合成效用。此外,对照实验证实了氧三醌中存在的环半酮的稳定性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
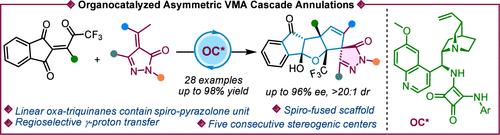
Enantioselective Vinylogous Michael Organocascade of COCF3-Tethered Enediones and Pyrazolinones: Access to Spiro-Fused Linear Oxa-Triquinanes
We report a streamlined organocatalytic enantioselective construction of spiro-fused polycyclic scaffolds, achieving excellent yields and diastereo- and enantioselectivities. A bifunctional squaramide catalyst enables an asymmetric VMA reaction, followed by a proton transfer/aldol/acetalization cascade of vinylogous pyrazolones with trifluoroacetyl-tethered enediones. Additionally, the scalability and postsynthetic transformations of the titled products highlight their synthetic utility. Furthermore, control experiments confirmed the stability of the cyclic hemiketals present in the oxa-triquinanes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


