胰岛素信号网络
IF 20.8
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
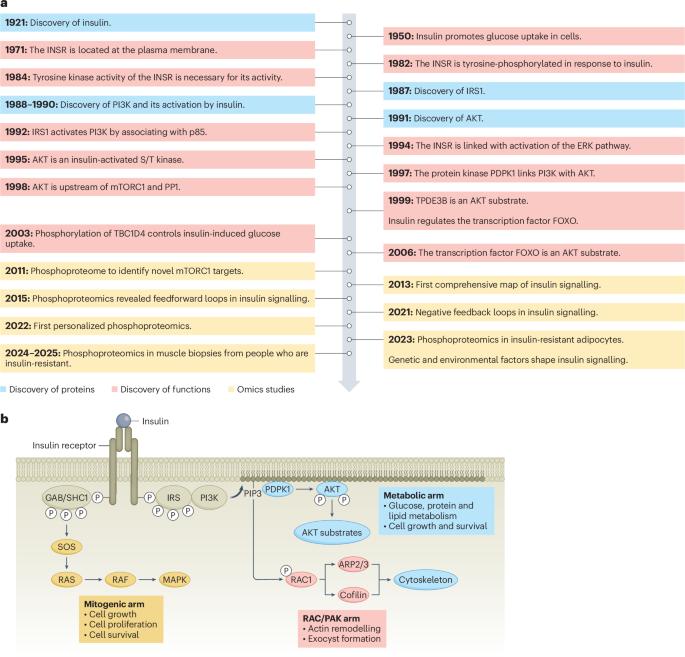
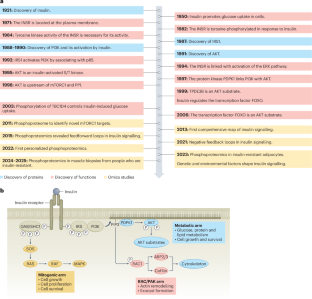
胰岛素信号是代谢的中心调节器,协调营养稳态和碳水化合物、蛋白质和脂质代谢。该网络通过动态、严格调控的蛋白磷酸化事件运作,涉及关键激酶,如AKT,以惊人的精度塑造细胞反应。磷蛋白组学的进展扩大了我们对胰岛素信号的理解,揭示了其复杂的调控及其与疾病,特别是心脏代谢疾病的联系。主要的见解,如AKT的激活机制和遗传和环境因素的影响,已经从研究这个网络中出现。在这篇综述中,我们研究了胰岛素信号的结构,重点是其精确的时间调节。我们强调AKT在胰岛素作用中的核心作用及其广泛的底物库,它控制着多种细胞功能。此外,我们还探索了反馈和串音机制,如胰岛素受体底物蛋白信号,它通过数百个不同位点的磷酸化整合输入。至关重要的是,磷酸化蛋白质组学揭示了胰岛素抵抗状态的复杂性,其中网络重新布线的特征是磷酸化被破坏和健康细胞中不存在的新位点的出现。这些见解重新定义了胰岛素信号传导及其功能障碍,突出了新的治疗机会。本文章由计算机程序翻译,如有差异,请以英文原文为准。
The insulin signalling network
Insulin signalling is a central regulator of metabolism, orchestrating nutrient homeostasis and coordinating carbohydrate, protein and lipid metabolism. This network operates through dynamic, tightly regulated protein phosphorylation events involving key kinases such as AKT, shaping cellular responses with remarkable precision. Advances in phosphoproteomics have expanded our understanding of insulin signalling, revealing its intricate regulation and links to disease, particularly cardiometabolic disease. Major insights, such as the mechanisms of AKT activation and the influence of genetic and environmental factors, have emerged from studying this network. In this Review, we examine the architecture of insulin signalling, focusing on its precise temporal regulation. We highlight AKT’s central role in insulin action and its vast substrate repertoire, which governs diverse cellular functions. Additionally, we explore feedback and crosstalk mechanisms, such as insulin receptor substrate protein signalling, which integrates inputs through phosphorylation at hundreds of distinct sites. Crucially, phosphoproteomics has uncovered complexities in insulin-resistant states, where network rewiring is characterized by disrupted phosphorylation and the emergence of novel sites that are absent in healthy cells. These insights redefine insulin signalling and its dysfunction, highlighting new therapeutic opportunities. In this Review, the authors provide a detailed overview of insulin signalling and its dysfunction, highlighting new therapeutic opportunities.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


