d-半胱氨酸通过抑制半胱氨酸脱硫酶NFS1来损害肿瘤生长
IF 20.8
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
肿瘤细胞的选择性靶向是癌症治疗的主要挑战。许多癌细胞过度表达胱氨酸/谷氨酸反转运蛋白xCT/CD98,这是一种l-胱氨酸转运系统,可增强抗氧化防御,从而促进肿瘤的生存和进展。在这里,我们发现半胱氨酸的d-对映体(d-Cys)被选择性地导入到过表达xCT/ cd98的癌细胞系中,并损害它们的增殖,特别是在高氧浓度下。细胞内d-Cys特异性抑制线粒体半胱氨酸脱硫酶NFS1, NFS1是细胞铁硫蛋白生物生成的关键酶,通过阻断硫的位阻动员。d-Cys对NFS1的抑制影响所有细胞铁硫团依赖的功能,包括线粒体呼吸、核苷酸代谢和基因组完整性的维持,导致氧气消耗减少、DNA损伤和细胞周期停滞。d-Cys可抑制小鼠乳腺原位植入人三阴性乳腺癌细胞的肿瘤生长。因此,d-Cys可能是一种简单的治疗方法,可以选择性地靶向那些以xCT/CD98过表达为特征的癌症。本文章由计算机程序翻译,如有差异,请以英文原文为准。
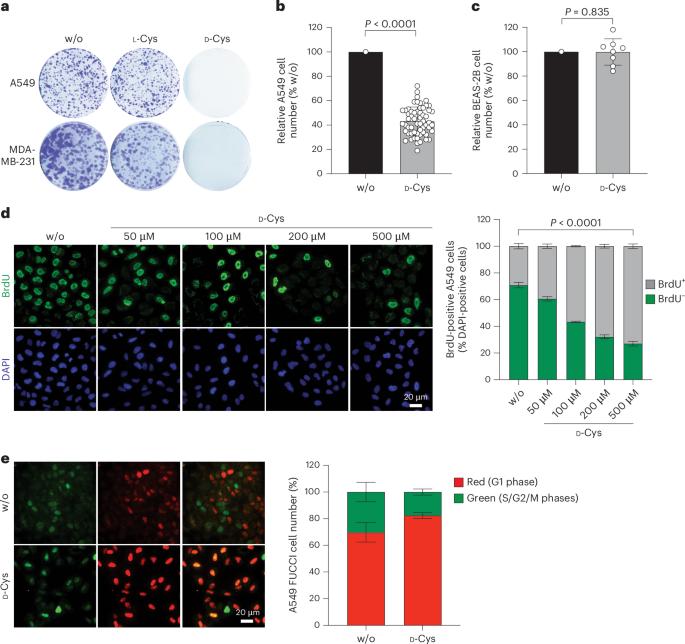
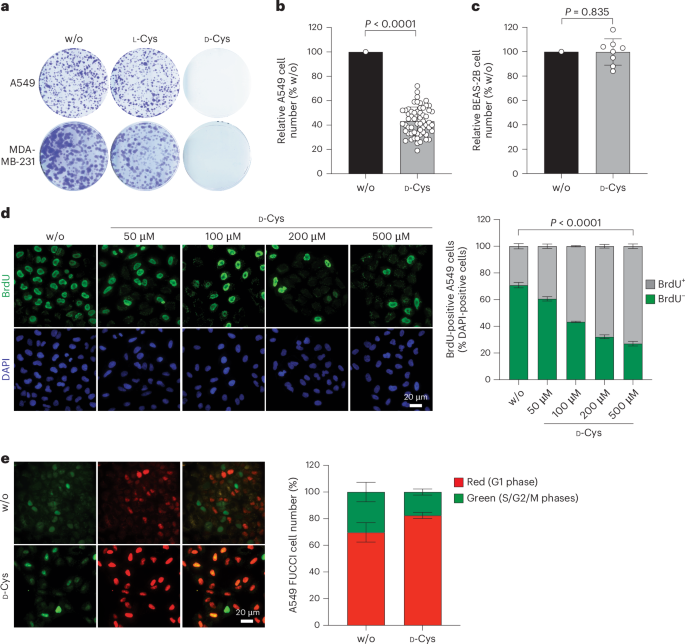
d-cysteine impairs tumour growth by inhibiting cysteine desulfurase NFS1
Selective targeting of cancer cells is a major challenge for cancer therapy. Many cancer cells overexpress the cystine/glutamate antiporter xCT/CD98, an l-cystine transport system that strengthens antioxidant defences, thereby promoting tumour survival and progression. Here, we show that the d-enantiomer of cysteine (d-Cys) is selectively imported into xCT/CD98-overexpressing cancer cell lines and impairs their proliferation, particularly under high oxygen concentrations. Intracellular d-Cys specifically inhibits the mitochondrial cysteine desulfurase NFS1, a key enzyme of cellular iron–sulfur protein biogenesis, by blocking sulfur mobilization due to steric constraints. NFS1 inhibition by d-Cys affects all cellular iron–sulfur cluster-dependent functions, including mitochondrial respiration, nucleotide metabolism and maintenance of genome integrity, leading to decreased oxygen consumption, DNA damage and cell cycle arrest. d-Cys administration diminishes tumour growth of human triple-negative breast cancer cells implanted orthotopically into the mouse mammary gland. Hence, d-Cys could represent a simple therapy to selectively target those forms of cancer characterized by overexpression of xCT/CD98. Zangari et al. show that d-cysteine targets NFS1, thus affecting Fe–S cluster biogenesis and impairing tumour growth.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


