1-脱氧- d -木糖5-磷酸合成酶:一类类异戊二烯生物合成必需酶的结构研究。
IF 2.7
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
类异戊二烯是最大的、功能多样的天然产物之一,在生命所有领域的细胞过程中发挥着重要作用。与人类不同,许多致病生物如细菌和原生动物通过2- c -甲基赤藓糖醇磷酸(MEP)途径产生类异戊二烯前体。1-脱氧-d -木糖5-磷酸合酶(DXPS)是该途径的第一酶和限速酶。尽管DXPS具有重要的生物学意义和作为药物靶点的潜力,但由于其固有的灵活性和结晶困难,对DXPS的结构研究受到限制。最近的进展,包括更多结晶友好结构的发展和单粒子冷冻电子显微镜(cryo-EM)的应用,极大地扩展了我们对DXPS的结构理解。这篇综述提供了一个全面的概述,在过去的几十年里获得的结构见解,重点是DXPS的整体结构,它的催化机制,并在基于结构的药物发现新兴的相关性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
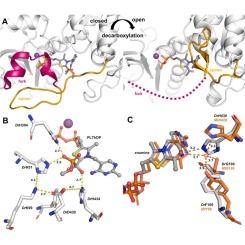
1-Deoxy-D-xylulose 5-phosphate synthase: structural perspectives on an essential enzyme in isoprenoid biosynthesis
Isoprenoids represent one of the largest and functionally diverse class of natural products, playing essential roles in cellular processes across all domains of life. Unlike humans, many pathogenic organisms such as bacteria and protozoa produce their isoprenoid precursors through the 2-C-methylerythritol phosphate (MEP) pathway. 1-deoxy-D-xylulose 5-phosphate synthase (DXPS) is the first and rate-limiting enzyme of this pathway. Despite its biological importance and potential as a drug target, structural studies on DXPS were limited due to its intrinsic flexibility and difficulties in crystallisation. Recent advances, including the development of more crystallisation-friendly constructs and the application of single-particle cryo-electron microscopy (cryo-EM), have significantly expanded our structural understanding of DXPS. This review provides a comprehensive overview of the structural insights gained over the past decades, focusing on the overall architecture of DXPS, its catalytic mechanism, and emerging relevance in structure-based drug discovery.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of structural biology
生物-生化与分子生物学
CiteScore
6.30
自引率
3.30%
发文量
88
审稿时长
65 days
期刊介绍:
Journal of Structural Biology (JSB) has an open access mirror journal, the Journal of Structural Biology: X (JSBX), sharing the same aims and scope, editorial team, submission system and rigorous peer review. Since both journals share the same editorial system, you may submit your manuscript via either journal homepage. You will be prompted during submission (and revision) to choose in which to publish your article. The editors and reviewers are not aware of the choice you made until the article has been published online. JSB and JSBX publish papers dealing with the structural analysis of living material at every level of organization by all methods that lead to an understanding of biological function in terms of molecular and supermolecular structure.
Techniques covered include:
• Light microscopy including confocal microscopy
• All types of electron microscopy
• X-ray diffraction
• Nuclear magnetic resonance
• Scanning force microscopy, scanning probe microscopy, and tunneling microscopy
• Digital image processing
• Computational insights into structure
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


