新型小分子类似物促进Apelin受体拮抗,增强血浆和微粒体稳定性。
IF 2.2
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
APJ是一种G蛋白偶联受体,由于其在肿瘤生长、血管生成、转移和抗vegf治疗中的作用,已成为肿瘤治疗中有希望的治疗靶点。然而,唯一报道的小分子APJ拮抗剂ML221表现出极差的血浆(1/2 50 = 3.1 μM和3.2 μM;抑制率分别为90.1 %和80.1 %),同时代谢稳定性显著增强(1 h后血浆中仍保持100% %;肝微粒体t1/2 = 5 min)。这些化合物选择性地抑制过表达apj的癌细胞(OVCAR8)和抑制apelin诱导的内皮细胞迁移(OVCAR3和HUVEC),其中化合物21对细胞迁移的抑制作用最强。总的来说,本研究确定了磺酸键是一种特别有利的支架修饰,也是一种代谢稳定的酯类生物同位体,可以在不影响APJ拮抗的情况下增强稳定性。这些类似物作为下一代APJ拮抗剂用于癌症治疗,具有进一步体内优化和进展的前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
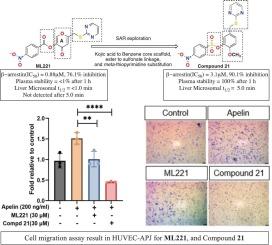
Novel small molecule analogs for advancing apelin receptor antagonism with enhanced plasma and microsomal stability
The apelin receptor (APJ), a G protein-coupled receptor, has emerged as a promising therapeutic target in oncology due to its role in tumor growth, angiogenesis, metastasis, and resistance to anti-VEGF therapy. However, ML221, the only reported small-molecule APJ antagonist, exhibits extremely poor plasma (<1 % remaining after 1 h) and liver microsomal (t1/2 < 1 min) stability, limiting its translational potential. To address this, we designed and synthesized a focused series of ML221 analogs incorporating amide, ether, and sulfonate linkages to block esterase-mediated degradation aiming to improve metabolic stability while maintaining APJ antagonistic activity. Among them, sulfonate-linked analogs 21 and 22 retained strong β-arrestin inhibition (IC50 = 3.1 μM and 3.2 μM; 90.1 % and 80.1 % inhibition, respectively) while exhibiting significantly enhanced metabolic stability (100 % remaining in plasma after 1 h; liver microsomal t1/2 = 5 min). These compounds selectively inhibited APJ-overexpressing cancer cells (OVCAR8) and suppressed apelin-induced endothelial cell migration (OVCAR3 and HUVEC), with compound 21 showing the most potent inhibition of cell migration. Overall, this study establishes the sulfonate linkage as a particularly favorable scaffold modification and a metabolically stable ester bioisostere that enhances stability without compromising APJ antagonism. These analogs represent promising candidate for further in-vivo optimization and advancement as next-generation APJ antagonists for cancer therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.70
自引率
3.70%
发文量
463
审稿时长
27 days
期刊介绍:
Bioorganic & Medicinal Chemistry Letters presents preliminary experimental or theoretical research results of outstanding significance and timeliness on all aspects of science at the interface of chemistry and biology and on major advances in drug design and development. The journal publishes articles in the form of communications reporting experimental or theoretical results of special interest, and strives to provide maximum dissemination to a large, international audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


