化学选择性氢胺甲基化的Dirhodium(ii,ii)催化剂优化:迈向高效胺合成
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
利用四种已合成的异电性dihodium (II,II) acetato-bipyridyl络合物(1-4),对亚胺和胺的化学选择性加氢混合配体dihodium (II,II)催化剂进行了优化。因此,优化反应条件,包括温度、H2压力、催化剂负载和反应时间的变化,应用于模型底物,以确定对目标胺产物的选择性。在优化的加氢反应条件下对标题配合物进行了评价,三氟甲基取代配合物(3)在该系列中表现出最高的加氢活性。改变合成气混合物中CO相对于H2的分压,以优化烯烃底物转化为目标胺产品,然后使用催化剂前驱体(3)。胺类反应物多种多样,芳香胺的使用导致目标产物的转化率较低,而脂肪胺底物与一系列烯烃结合可以很好地生产仲胺和叔胺。在优化的催化剂和反应条件下,使用催化剂前体3对合适的底物进行氢胺甲基化反应,得到两种已知阿片类镇痛药曲马多®的类似物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
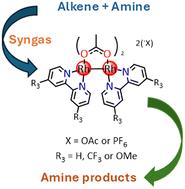
Dirhodium(ii,ii) catalyst optimisation for chemoselective hydroaminomethylation: towards efficient amine synthesis†
The optimisation of a suitable mixed ligand dirhodium(ii,ii) catalyst for chemoselective hydrogenation of imines and enamines was carried out using four previously synthesised heteroleptic dirhodium(ii,ii) acetato-bipyridyl complexes (). As such, optimised reaction conditions incorporating changes in temperature, H2 pressure, catalyst loading, and reaction time were applied to model substrates to determine the selectivity towards target amine product(s). The title complexes were evaluated under the optimised conditions for the hydrogenation reaction with the trifluoromethyl substituted complex () showing the highest hydrogenation activity for the series. Varying the partial pressure of CO relative to H2 in the syngas mixture to optimise the conversion of olefin substrate to form the target amine products was then applied with catalyst precursor (). The amine reactants were varied, and the use of aromatic amines resulted in low conversion to target products, while aliphatic amine substrates showed good to excellent production of both secondary and tertiary amines in combination with a range of olefins. With the optimised catalyst and reaction conditions in hand, the hydroaminomethylation of a suitable substrate using catalyst precursor to afford two analogues of a known opioid analgesic, Tramadol® was carried out.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


