固定化离子液体-金属离子共催化剂协同催化氯烷脱氢氯化与乙炔加氢氯化:DFT研究
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
明显的HCl吸附严重阻碍了卤化烃的催化脱氢氯化,导致催化剂降解或失活。将这一过程与消耗盐酸的乙炔加氢氯化相结合,提供了一种有效的策略来缓解这一问题。采用PBE0/ma-TZVP水平的密度泛函理论(DFT)模拟研究了一系列金属氯化物及其Cl -配合物对反应物的吸附。基于吸附能数据,筛选了多种不同特性的共催化剂,并对其催化机理进行了系统研究。kcl - cucl离子液体催化剂在理论上被证明是最佳催化剂,通过离子液体和金属盐的不同解离状态,促进氯烷脱氢氯化反应(ΔG = 134.0 kJ mol−1)和乙炔加氢氯化反应(ΔG = 124.4 kJ mol−1)。此外,KCl抑制多重乙炔吸附,加速烯烃解吸,阻碍乙炔铜的形成,从而确保持续的催化稳定性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
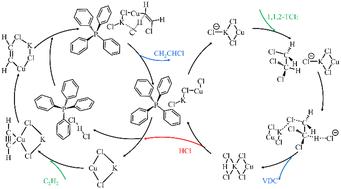
Synergistic catalysis of chloroalkane dehydrochlorination with acetylene hydrochlorination by immobilized ionic liquid–metal ion co-catalysts: a DFT study†
Pronounced adsorption of HCl critically hinders the catalytic dehydrochlorination of halogenated hydrocarbons, leading to catalyst degradation or deactivation. Coupling this process with HCl-consuming acetylene hydrochlorination offers an effective strategy to mitigate this issue. Density functional theory (DFT) simulations at the PBE0/ma-TZVP level are employed to investigate the adsorption of reactants on a series of metal chlorides and their Cl− complexes. Based on the adsorption energy data, a variety of co-catalysts with different characteristics are screened and their catalytic mechanisms are systematically investigated. The KCl–CuCl–ionic liquid catalyst has been theoretically proven to be the optimal catalyst, facilitating the dehydrochlorination of chloroalkanes (ΔG = 134.0 kJ mol−1) and acetylene hydrochlorination (ΔG = 124.4 kJ mol−1) through the different dissociation states of ionic liquids and metal salts. Furthermore, KCl inhibits multiple acetylene adsorption, accelerates olefin desorption, and hinders the formation of copper acetylide, thereby ensuring sustained catalytic stability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


