配体辅助镍催化酰基肼的N,N-二烷基化和脂肪醇环化
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在这里,我们描述了一个镍催化的酰基酰肼的N,N-二酰化方案。一系列脂肪醇和二醇成功脱氢,并用于这一具有挑战性的二烷基化反应以及一些环化反应。该反应具有化学选择性,因为许多N,N-二烷基化产物含有一个烯烃基序,亚胺键选择性地减少,而烯烃键保持完整。偶氮-腙氧化还原对亚胺的加氢反应起着至关重要的作用。该反应通过自由基途径进行,与之前报道的涉及金属氢化物的反应形成鲜明对比。本文章由计算机程序翻译,如有差异,请以英文原文为准。
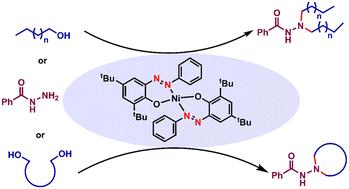
Ligand-assisted nickel catalysis enabling N,N-dialkylation and cyclization of acyl hydrazides using aliphatic alcohols†
Herein, we describe a nickel-catalyzed N,N-dilakylation protocol for acyl hydrazides. A series of aliphatic alcohols and diols were successfully dehydrogenated and used for this challenging dialkylation as well as some cyclization reactions. The reaction is chemoselective, as many of the N,N-dialkylated products contain an olefinic motif, with the imine linkage selectively reduced while the olefinic one remains intact. The azo–hydrazo redox couple plays a crucial role in the hydrogenation of imines. The reaction proceeds via a radical pathway and in striking contrast to previous reports that involve metal hydrides.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


