可回收铜(i)催化生物衍生2-甲基糠醛中1-溴-2-碘苯和β-酮酯的偶联:2,3-二取代苯并呋喃的绿色合成
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
以1,1′-(丁烷-1,4-二基)二(3-(3-(三乙氧基硅基)丙基)- 1h -咪唑-3-铵)氯为载体,在SBA-15上固定化制备了介孔SBA-15锚定的n -杂环碳(NHC)-铜(I)配合物[SBA-15-NHC-CuI],并与CuI和NaOtBu反应。以SBA-15-NHC-CuI (10 mol% Cu)为催化剂,1-溴-2-碘苯和β-酮酯在110°C下以K2CO3为底物在生物衍生的2- methf中顺利进行了骨牌C-C /C - o偶联反应,得到了多种2,3-二取代苯并呋喃,产率高,官能团耐受性广。这种新型的非均相nhc -铜(I)催化剂可以通过离心反应混合物很容易地回收,并且在没有明显的催化活性损失的情况下可以循环使用8次。本文章由计算机程序翻译,如有差异,请以英文原文为准。
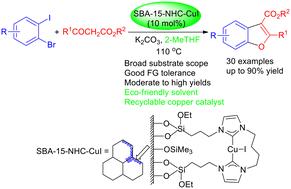
Recyclable copper(i)-catalyzed coupling of 1-bromo-2-iodobenzenes and β-keto esters in bioderived 2-MeTHF: green synthesis of 2,3-disubstituted benzofurans†
A mesoporous SBA-15-anchored N-heterocyclic carbene (NHC)-copper(i) complex [SBA-15-NHC-CuI] was prepared via immobilization of 1,1′-(butane-1,4-diyl)bis(3-(3-(triethoxysilyl)propyl)-1H-imidazol-3-ium) chloride onto SBA-15, followed by reaction with CuI and NaOtBu. With the use of SBA-15-NHC-CuI (10 mol% Cu) as a catalyst, the domino C–C/C–O coupling reaction between 1-bromo-2-iodobenzenes and β-keto esters proceeded smoothly in bioderived 2-MeTHF at 110 °C with K2CO3 as a base to deliver a wide array of 2,3-disubstituted benzofurans in good to high yields with a wide tolerance of functional groups. This new heterogenized NHC-copper(i) catalyst could be facilely recovered by centrifugation of the reaction mixture and recycled up to eight cycles without a significant loss of catalytic activity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


