单电子转移(SET)和碘原子转移自由基加成(I-ATRA)诱导环丙化反应:阐明碘†的作用
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
采用离散傅里叶变换(DFT)研究了以苯基有机烃(PLY)为催化剂,碘为共催化剂,烯烃与重氮乙酸在可见光下的环丙化反应机理。在没有碘的情况下,系统在芳基硫醇的帮助下进行氢烷基化反应。研究了这两个反应,以了解碘在促进环丙烷形成中的作用,环丙烷是药效团的关键结构单元。我们的研究结果表明,单电子转移(SET)会产生短暂的碘自由基,而碘原子转移自由基加成(I-ATRA)会为环丙化反应构建碘化乙酸基段。另一方面,质子耦合电子转移(PCET)机制诱导了氢烷基化反应中质子化乙酸自由基的形成。碘的孤对轨道向新生成的C-C键的反键轨道的反向捐赠可以忽略不计,这使得碘成为更好的离去基,从而使环化成为可能,验证了碘在环丙化反应中的作用。分子内原子分析、激活应变分析和能量分解分析等多种分析技术表明,有利的非共价相互作用和空间效应促进了区域选择性的反马尔可夫尼科夫自由基加成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
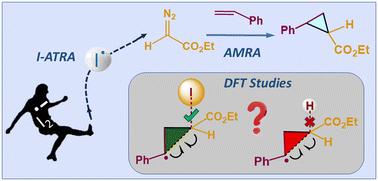
Single electron transfer (SET) and iodine-atom transfer radical addition (I-ATRA) induced cyclopropanation reaction: elucidating the role of iodine†
Mechanistic studies were conducted for the visible-light-mediated cyclopropanation reaction of alkenes with diazoacetate, catalyzed by a phenalenyl-based organic hydrocarbon (PLY) and co-catalysed by iodine, using DFT. In the absence of iodine, the system proceeds towards the hydroalkylation reaction, with the aid of aryl thiol. Both reactions were studied to understand the role of iodine in promoting the formation of cyclopropanes, the key structural units in pharmacophores. Our findings suggest that the single electron transfer (SET) will generate the transient iodine radical species, whereas the iodine atom transfer radical addition (I-ATRA) will construct the iodinated acetate radical moiety for the cyclopropanation reaction. On the other hand, the proton-coupled electron transfer (PCET) mechanism induces the formation of a protonated acetate radical for the hydroalkylation reaction. The negligible back donation from the lone pair orbital of iodine to the antibonding orbital of the newly generated C–C bond makes the iodine a better leaving group, thereby making the cyclisation feasible, validating the role of iodine in the cyclopropanation reaction. Various analysis techniques such as atoms in molecule, activation strain analysis, and energy decomposition analysis reveal that the favorable non-covalent interactions and steric effects promote the regioselective anti-Markovnikov radical addition over the Markovnikov radical addition.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


