绘制三苯硼烷(BPh3)催化co2 -环氧化物偶联到碳酸盐的催化景观:一种解决底物依赖选择性的硅方法
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
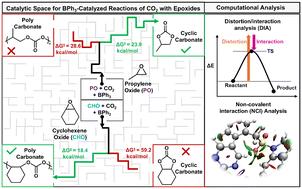
二氧化碳与环氧化物的催化偶联是一种很有前途的碳增值方法,可以合成环碳酸盐(CCs)和聚碳酸盐(PCs)。然而,控制产品的选择性仍然是一个挑战。三苯硼烷(BPh3)已成为一种很有前途的无金属催化剂,但其底物依赖性选择性的起源仍不清楚。虽然BPh3选择性地与环氧丙烷(PO)形成CCs,但它只与环氧环己烯(CHO)产生pc,突出了独特的反应性。为了理解这种选择性,我们进行了一项全面的密度泛函理论(DFT)研究,绘制了bph3介导的二氧化碳-环氧化物偶联的催化景观,并将其与缺乏这种选择性的三乙基硼烷(BEt3)催化进行了比较。计算结果表明,环氧化物开环是反应速率的决定步骤,与实验结果一致。此外,高浓度CO2可以形成抑制环氧化物活化的非活性物质,这解释了实验观察到的对CO2浓度的反比依赖。我们的畸变/相互作用分析(DIA)和非共价相互作用(NCI)分析表明,在bph3催化的CO2和PO偶联中,环氧化物加成步骤中较弱的分子间相互作用不利于PC的形成,而有利于CC的形成。相反,对于CO2和CHO耦合,合环阶段的高畸变能不利于CC的形成,导致PC成为主导产物。相比之下,BEt3催化稳定了两种环氧化物之间PC的形成,消除了选择性。本研究概述了bph3催化CO2-环氧化物偶联的催化景观,揭示了硼取代如何控制选择性,并为设计选择性CO2利用的硼基催化剂提供了见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Mapping the catalytic landscape of triphenylborane (BPh3)-catalyzed CO2-epoxide coupling to carbonates: an in silico approach to solve substrate-dependent selectivity†
The catalytic coupling of CO2 and epoxides is a promising approach for carbon valorization, enabling the synthesis of cyclic-carbonates (CCs) and poly-carbonates (PCs). However, controlling product selectivity remains a challenge. Triphenylborane (BPh3) has emerged as a promising metal-free catalyst, yet the origins of its substrate-dependent selectivity remain unclear. While BPh3 selectively forms CCs with propylene oxide (PO), it exclusively produces PCs with cyclohexene oxide (CHO), highlighting distinct reactivity. To understand this selectivity, we conducted a comprehensive density functional theory (DFT) study, mapping the catalytic landscape of BPh3-mediated CO2-epoxide coupling and comparing it with triethylborane (BEt3) catalysis, which lacks such selectivity. Our calculations reveal that epoxide ring opening is the rate-determining step, consistent with experimental studies. Additionally, high CO2 concentrations can form an inactive species that inhibits epoxide activation, explaining the experimentally observed inverse rate dependence on CO2 concentration. Our distortion/interaction analysis (DIA) and non-covalent interaction (NCI) analysis show that in BPh3-catalyzed CO2 and PO coupling, weaker intermolecular interactions in the epoxide addition step disfavour PC formation, favoring CC formation. Conversely, for CO2 and CHO coupling, the high distortion energy in the ring-closing step makes CC formation unfavourable, leading to PC as the dominant product. In contrast, BEt3 catalysis stabilizes PC formation across both epoxides, eliminating selectivity. This study sketches the catalytic landscape of BPh3-catalyzed CO2-epoxide coupling, revealing how boron substitution governs selectivity and offers insights for designing boron-based catalysts for selective CO2 utilization.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


