钯催化烯烯烯氧化碳环化-烯烃辅助硼化的机理和选择性研究
IF 3
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
通过密度泛函理论计算,对pd催化烯烯烯氧化碳环化硼化反应机理进行了系统的理论研究。我们的计算分析表明,催化循环包括四个主要阶段,包括CH键激活、烯烃插入、金属转化和还原消除。反应通过协同的金属-去质子化途径通过C(sp3)H活化开始,产生乙烯基钯中间体。立体化学控制是由烯烃插入的区域选择性控制的:近端插入导致螺旋环产物,而远端插入优先形成环己烯产物,后者在动力学和热力学上都表现出更有利的途径。根据亲核试剂的哪个氧原子攻击,金属转化步骤遵循四元或六元环过渡态的两条路径。发生还原消除,以苯醌为氧化剂再生催化剂,完成催化循环。不同CC耦合途径的对比分析进一步验证了所提出的机理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
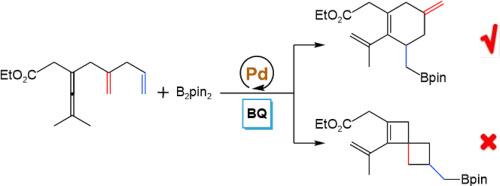
Insights into mechanism and selectivity in palladium-catalyzed oxidative carbocyclization-borylation of enallenes via olefin assistance
A systematic theoretical study has been carried out with the aid of density functional theory calculations on the mechanism for the Pd-catalyzed oxidative carbocyclization-borylation of enallenes. Our computational analysis reveals that the catalytic cycle comprises four principal stages, including C![]() H bond activation, olefin insertion, transmetalation, and reductive elimination. The reaction initiates via C(sp3)
H bond activation, olefin insertion, transmetalation, and reductive elimination. The reaction initiates via C(sp3)![]() H activation through a concerted metalation-deprotonation pathway, generating a vinylpalladium intermediate. Stereochemical control is governed by the regioselectivity of alkene insertion: proximal insertion leads to spirocyclic products, whereas distal insertion preferentially forms cyclohexene products, with the latter pathway exhibiting more favorable in both kinetics and thermodynamics. The transmetalation step follows two paths through four- or six-membered ring transition states, depending on which oxygen atom of the nucleophile attacks. Reductive elimination occurs and the catalyst is regenerated using benzoquinone as the oxidant to complete the catalytic cycle. Comparative analysis of alternative C
H activation through a concerted metalation-deprotonation pathway, generating a vinylpalladium intermediate. Stereochemical control is governed by the regioselectivity of alkene insertion: proximal insertion leads to spirocyclic products, whereas distal insertion preferentially forms cyclohexene products, with the latter pathway exhibiting more favorable in both kinetics and thermodynamics. The transmetalation step follows two paths through four- or six-membered ring transition states, depending on which oxygen atom of the nucleophile attacks. Reductive elimination occurs and the catalyst is regenerated using benzoquinone as the oxidant to complete the catalytic cycle. Comparative analysis of alternative C![]() C coupling pathways further validates the proposed mechanism.
C coupling pathways further validates the proposed mechanism.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Computational and Theoretical Chemistry
CHEMISTRY, PHYSICAL-
CiteScore
4.20
自引率
10.70%
发文量
331
审稿时长
31 days
期刊介绍:
Computational and Theoretical Chemistry publishes high quality, original reports of significance in computational and theoretical chemistry including those that deal with problems of structure, properties, energetics, weak interactions, reaction mechanisms, catalysis, and reaction rates involving atoms, molecules, clusters, surfaces, and bulk matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


