脂肪组织特异性Yap基因敲除通过抑制脂肪分解加剧饮食诱导的肥胖。
IF 3.6
3区 医学
Q2 CELL BIOLOGY
引用次数: 0
摘要
背景和目的:YAP调节多种细胞过程,包括细胞接触抑制、机械转导、细胞分化和增殖、细胞凋亡和癌症进展。虽然YAP在体外抑制脂肪形成,但其在肥胖中的作用尚未完全阐明。方法和结果:在本研究中,我们建立了脂肪组织特异性YapaKO敲除小鼠模型(YapaKO),发现与对照小鼠相比,雄性(而非雌性)YapaKO小鼠表现出更高的高脂肪饮食诱导的肥胖表型。从机制上讲,这种影响可能是由于抑制了脂溶活性,这是由甘油三酯脂溶酶(包括ATGL和HSL)的表达减少引起的。抑制脂溶活性导致禁食期间循环游离脂肪酸水平降低,使雄性小鼠在冷暴露后无法维持核心体温,并在禁食状态下表现出运动能力受损。本研究揭示了YAP在控制脂肪分解中的新作用。结论:YAP是脂肪组织中脂肪分解的生理调节剂。在脂肪组织中激活YAP可能促进脂肪分解和减少肥胖。本文章由计算机程序翻译,如有差异,请以英文原文为准。
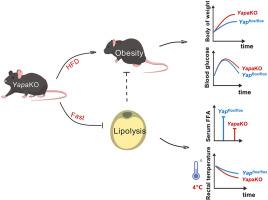
Adipose tissue-specific Yap knockout exacerbates diet-induced obesity through suppression of lipolysis
Background and aims
YAP regulates various cellular processes, including cell contact inhibition, mechanotransduction, cell differentiation and proliferation, apoptosis, and cancer progression. Although YAP suppresses adipogenesis in vitro, its role in obesity has not yet been completely elucidated.
Methods and results
In this study, we generated an adipose tissue-specific Yap knockout mouse model (YapaKO), and found that male, but not female, YapaKO mice showed an enhanced high-fat diet-induced obesity phenotype compared to control mice. Mechanistically, this effect is potentially due to suppressed lipolytic activity, which results from the decreased expression of triglyceride lipolytic enzymes, including ATGL and HSL. The inhibition of lipolytic activity led to reduced levels of circulating free fatty acids during fasting, making male mice unable to maintain core body temperature after cold exposure and showing impaired exercise capability in the fasted state. This study reveals a novel role of YAP in controlling lipolysis.
Conclusion
YAP is a physiological regulator of lipolysis in the adipose tissue. YAP activation in adipose tissue may facilitate lipolysis and reduce obesity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular and Cellular Endocrinology
医学-内分泌学与代谢
CiteScore
9.00
自引率
2.40%
发文量
174
审稿时长
42 days
期刊介绍:
Molecular and Cellular Endocrinology was established in 1974 to meet the demand for integrated publication on all aspects related to the genetic and biochemical effects, synthesis and secretions of extracellular signals (hormones, neurotransmitters, etc.) and to the understanding of cellular regulatory mechanisms involved in hormonal control.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


