LIMK2通过激活mst4介导的NPM1磷酸化,促进中心体聚集和癌症进展。
IF 7.3
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
中心体扩增是多种恶性肿瘤的标志,它通过有丝分裂过程中的中心体聚集使癌细胞存活,为选择性消除具有多余中心体的癌细胞提供了一个有希望的治疗靶点。虽然中心体聚集的调控机制仍然知之甚少,但我们的研究确定了LIM激酶2 (LIMK2)是这一过程的关键调节因子,证明了癌症与肿瘤进展的相关性。机制上,LIMK2磷酸化哺乳动物不育-20样激酶4 (MST4)的苏氨酸178 (T178),激活其激酶功能。激活的MST4随后结合并磷酸化核磷蛋白1 (NPM1)的T95位点,这是中心体聚集和肿瘤细胞增殖所必需的修饰。NPM1基因缺失破坏中心体聚集并抑制恶性生长。体内研究显示,敲除LIMK2可显著减轻小鼠模型中4-硝基喹啉-1-氧化物(4NQO)诱导的食管肿瘤发生。通过shrna介导的下调或药物抑制(CRT0105950)靶向治疗LIMK2,通过防止“伪双极”纺锤体形成来抑制中心体聚集,诱导有丝分裂停止。中心体去簇化促进多极纺锤体组装,最终引发细胞凋亡。值得注意的是,CRT0105950治疗有效地抑制了细胞来源的异种移植物肿瘤的生长。我们的研究结果阐明了LIMK2/MST4/NPM1通路在癌症进展中的关键作用,并为广谱抗癌干预建立了新的治疗范式。本文章由计算机程序翻译,如有差异,请以英文原文为准。
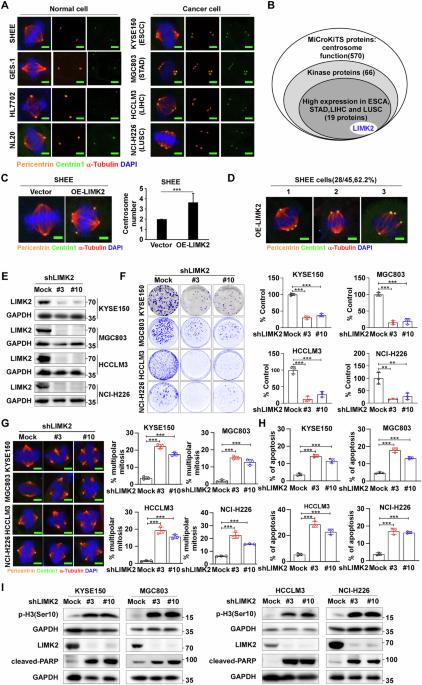
LIMK2 promotes centrosome clustering and cancer progression by activating MST4-mediated phosphorylation of NPM1
Centrosome amplification, a hallmark of diverse malignancies, enables cancer cell survival through centrosome clustering during mitosis, presenting a promising therapeutic target for selective elimination of cancer cells with supernumerary centrosomes. While the regulatory mechanisms underlying centrosome clustering remain poorly understood, our study identifies LIM kinase 2 (LIMK2) as a critical regulator of this process, demonstrating cancer correlation with tumor progression. Mechanistically, LIMK2 phosphorylates mammalian sterile-20-like kinase 4 (MST4) at threonine 178 (T178), activating its kinase function. Activated MST4 subsequently binds and phosphorylates nucleophosmin 1 (NPM1) at T95, a modification essential for centrosome clustering and tumor cell proliferation. Genetic depletion of NPM1 disrupts centrosome clustering and suppresses malignant growth. In vivo studies revealed that LIMK2 knockout significantly attenuates 4-nitroquinoline-1-oxide (4NQO) induced esophageal tumorigenesis in murine models. Therapeutic targeting of LIMK2 through shRNA-mediated knock down or pharmacological inhibition (CRT0105950) suppresses centrosome clustering by preventing “pseudo-bipolar” spindle formation, inducing mitosis arrest. This centrosome de-clustering promotes multipolar spindle assembly, ultimately triggering apoptotic cell death. Notably, CRT0105950 treatment effectively suppressed cell-derived xenograft tumor growth. Our findings elucidate the pivotal role of the LIMK2/MST4/NPM1 pathway in cancer progression and establish a novel therapeutic paradigm for broad-spectrum anticancer intervention.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


