组蛋白甲基转移酶DOT1L与LSD1协同控制胚期MPN细胞分裂
IF 13.4
1区 医学
Q1 HEMATOLOGY
引用次数: 0
摘要
在骨髓增生性肿瘤(MPN)中,jak2突变克隆可能经历克隆进化和恶性转化的持久性仍然是一个挑战,因此迫切需要新的治疗方法来减缓克隆进化和进展到爆发期。LSD1 (KDM1A)抑制剂可减轻MPN的症状和克隆负担,但这些化合物是否对晚期疾病有效至今仍难以捉摸。通过染色质聚焦CRISPR-Cas9筛选,我们鉴定出组蛋白甲基转移酶DOT1L是LSD1药理学抑制下的合成致死靶标。DOT1L基因敲除损害了异种移植物的细胞适应性,减少了增殖,延长了存活时间。此外,DOT1L基因失活可使LSD1抑制剂敏感性增加100倍,导致TP53突变体爆炸期MPN细胞周期阻滞和凋亡诱导。在机制上,我们已经确定了DOT1L的一种新的非规范功能,它共同占据lsd1结合增强子,并有助于抑制独立于其酶活性的转录程序。DOT1L缺失与LSD1抑制剂共同激活肿瘤抑制程序,而药物抑制DOT1L的催化活性未能产生类似的效果。这些发现表明,通过蛋白质降解或RNA干扰来利用DOT1L靶向,而不是传统的酶抑制,可以提高LSD1抑制剂对胚期MPN的治疗效果。本文章由计算机程序翻译,如有差异,请以英文原文为准。
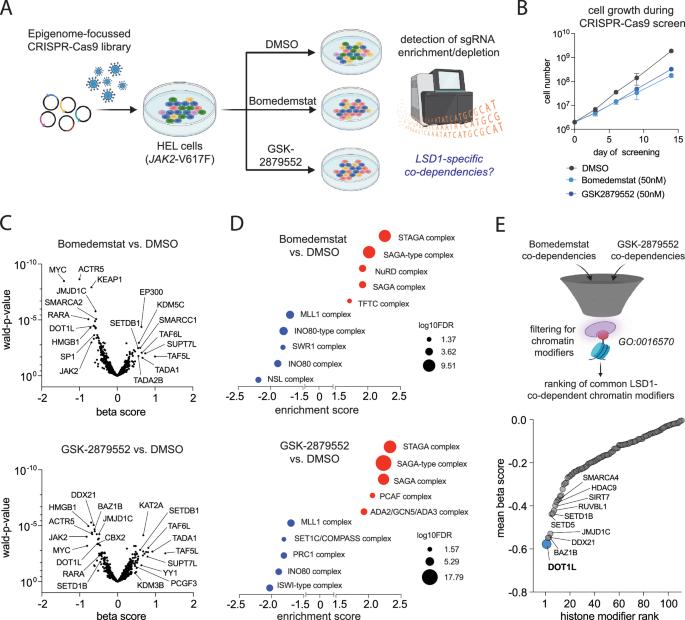
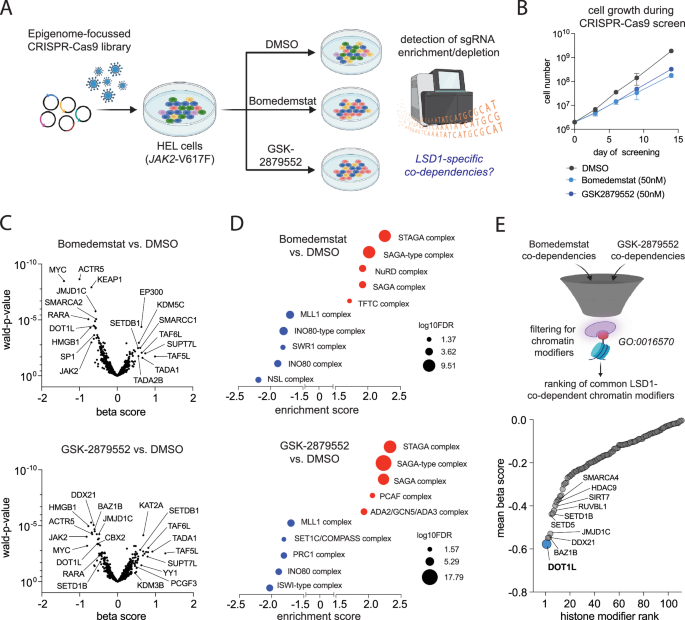
The histone-methyltransferase DOT1L cooperates with LSD1 to control cell division in blast-phase MPN
Persistence of JAK2-mutated clones that may undergo clonal evolution and malignant transformation remains a challenge in myeloproliferative neoplasms (MPN), Novel therapeutic approaches to attenuate clonal evolution and progression to blast-phase are therefore urgently needed. LSD1 (KDM1A) inhibitors reduce symptoms and clonal burden in MPN, but whether these compounds may be effective in advanced disease stages remained so far elusive. Using a chromatin-focused CRISPR-Cas9 screen, we identified the histone methyltransferase DOT1L as a synthetic lethal target under pharmacologic LSD1 inhibition. DOT1L knockout impaired cellular fitness, reduced proliferation, and prolonged survival in xenografts. Furthermore, genetic inactivation of DOT1L increased LSD1 inhibitor sensitivity up to 100-fold resulting in cell cycle arrest and apoptosis induction in TP53 mutant blast-phase MPN. Mechanistically, we have identified a novel, non-canonical function of DOT1L which co-occupied LSD1-bound enhancers and contributed to the repression of transcriptional programs independent of its enzymatic activity. DOT1L loss cooperated with LSD1 inhibitors to activate tumor suppressive programs, while pharmacologic inhibition of DOT1Ls catalytic activity failed to elicit comparable effects. These findings indicate that leveraging DOT1L targeting via protein degradation or RNA interference, rather than conventional enzymatic inhibition, could enhance the therapeutic efficacy of LSD1 inhibitors in blast-phase MPN.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


