嵌合双结构域溶酶的结构域相互作用导致广泛的杀菌活性。
IF 4.5
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
噬菌体衍生的溶酶是一种新型的、非传统的治疗多药耐药细菌的药物。然而,具有广泛宿主范围的工程裂解酶仍然没有得到很好的解决。先前,我们报道了嵌合lysin ClyR,它含有PlyC lysin的CHAP催化结构域(PlyCAC)和PlySs2 lysin的SH3b细胞壁结合结构域(PlySb),表现出扩大的宿主范围。然而,ClyR表现出扩大杀菌活性的机制仍不完全清楚。由于PlyCAC的结构是公开的(PDB代码:4F88),在这里,我们首先利用x射线衍射解算PlySb的晶体结构,然后利用各种生物物理方法,如x射线衍射、交联耦合质谱(CXMS)和小角度x射线散射(SAXS)分析ClyR的潜在全长结构。我们的研究结果表明,在两个组成域之间的多个域间相互作用的支持下,ClyR呈现出动态构象。诱变和生化分析进一步支持了这一观点,即这些结构域间的相互作用可能调节了ClyR的杀菌活性。总之,我们的研究结果提供了对ClyR作用机制的新见解,并更好地理解了域间相互作用如何影响嵌合溶酶的宿主范围。本文章由计算机程序翻译,如有差异,请以英文原文为准。
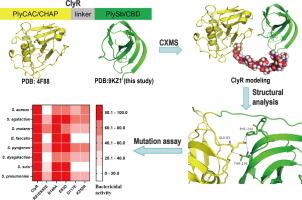
Domain Interactions in a Chimeric Dual-domain Lysin Lead to Broad Bactericidal Activity
Phage-derived lysins represent a novel, non-traditional therapeutic agent against multidrug-resistant bacteria. However, engineering lysins with broad host range remain poorly addressed. Previously, we reported that the chimeric lysin ClyR, which harbors the CHAP catalytic domain of PlyC lysin (PlyCAC) and the SH3b cell-wall binding domain of PlySs2 lysin (PlySb), exhibits an expanded host range. However, the mechanism by which ClyR exhibits expanded bactericidal activity is still not fully understood. Since the structure of PlyCAC is publicly available (PDB code: 4F88), here, we first solve the crystal structure of PlySb using X-ray diffraction, and then use various biophysical methods, such as X-ray diffraction, cross-linking coupled mass spectrometry (CXMS), and small-angle X-ray scattering (SAXS) to analyze the potential full-length structures of ClyR. Our results demonstrate that ClyR exhibits a dynamic conformation supported by multiple inter-domain interactions between the two constituent domains. Mutagenesis and biochemical analysis further support the notion that these inter-domain interactions may modulate the bactericidal activity of ClyR. Altogether, our findings provide novel insights into the action mechanism of ClyR and a better understanding of how inter-domain interactions influence the host range of chimeric lysins.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Biology
生物-生化与分子生物学
CiteScore
11.30
自引率
1.80%
发文量
412
审稿时长
28 days
期刊介绍:
Journal of Molecular Biology (JMB) provides high quality, comprehensive and broad coverage in all areas of molecular biology. The journal publishes original scientific research papers that provide mechanistic and functional insights and report a significant advance to the field. The journal encourages the submission of multidisciplinary studies that use complementary experimental and computational approaches to address challenging biological questions.
Research areas include but are not limited to: Biomolecular interactions, signaling networks, systems biology; Cell cycle, cell growth, cell differentiation; Cell death, autophagy; Cell signaling and regulation; Chemical biology; Computational biology, in combination with experimental studies; DNA replication, repair, and recombination; Development, regenerative biology, mechanistic and functional studies of stem cells; Epigenetics, chromatin structure and function; Gene expression; Membrane processes, cell surface proteins and cell-cell interactions; Methodological advances, both experimental and theoretical, including databases; Microbiology, virology, and interactions with the host or environment; Microbiota mechanistic and functional studies; Nuclear organization; Post-translational modifications, proteomics; Processing and function of biologically important macromolecules and complexes; Molecular basis of disease; RNA processing, structure and functions of non-coding RNAs, transcription; Sorting, spatiotemporal organization, trafficking; Structural biology; Synthetic biology; Translation, protein folding, chaperones, protein degradation and quality control.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


