综合肿瘤免疫谱揭示了类肉瘤肾细胞癌中矛盾免疫敏感性的介质
IF 44.5
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
摘要
具有肉瘤样特征的肾细胞癌(sRCC)是一种高度侵袭性的肿瘤类型,但免疫检查点阻断(ICB)优先响应。为了更好地理解这种矛盾的免疫敏感性的微环境介质,我们对人类sRCC肿瘤进行了单细胞分析,并将其与透明细胞RCC (ccRCC)进行了比较,并在空间和大量转录组数据集中验证了3000多个RCC肿瘤。我们使用这些正交方法描述了sRCC中强大的免疫网络:sRCC中的肿瘤浸润T细胞更活跃,随后被耗尽,同时被CXCL13表达富集。与此同时,三级淋巴结构在sRCC中普遍存在,与体液免疫活性的功能富集平行。肿瘤克隆分析显示,sRCC中铁相关程序增加,呈现出潜在的脆弱性。我们进一步利用sRCC的矛盾生物学来获得基因组去分化特征(GDS),该特征虽然预后不良,但可以识别出最可能从ICB中受益的患者,包括队列和肿瘤类型。本文章由计算机程序翻译,如有差异,请以英文原文为准。
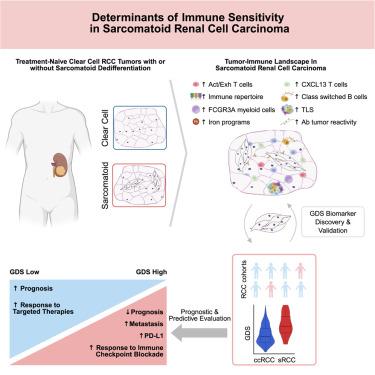
Comprehensive tumor-immune profiling reveals mediators of paradoxical immune sensitivity in sarcomatoid renal cell carcinoma
Renal cell carcinoma with sarcomatoid features (sRCC) is a highly aggressive tumor type yet preferentially responds to immune checkpoint blockade (ICB). To better understand microenvironmental mediators of this paradoxical immune sensitivity, we performed single-cell analyses of human sRCC tumors compared against clear cell RCC (ccRCC), with validation spatially and in bulk transcriptomic datasets totaling over 3,000 RCC tumors. We describe a robust immune network in sRCC using these orthogonal approaches: tumor-infiltrating T cells in sRCC are more activated, and subsequently exhausted, while being enriched for CXCL13 expression. Congruently, tertiary lymphoid structures are pervasive in sRCC, paralleling functional enrichment of humoral immune activity. Tumor clone analysis revealed increased iron-associated programs in sRCC, presenting a potential vulnerability. We furthermore leveraged the paradoxical biology of sRCC to derive a genomic dedifferentiation signature (GDS) that, while negatively prognostic, identifies patients most likely to benefit from ICB across cohorts and tumor types.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer Cell
医学-肿瘤学
CiteScore
55.20
自引率
1.20%
发文量
179
审稿时长
4-8 weeks
期刊介绍:
Cancer Cell is a journal that focuses on promoting major advances in cancer research and oncology. The primary criteria for considering manuscripts are as follows:
Major advances: Manuscripts should provide significant advancements in answering important questions related to naturally occurring cancers.
Translational research: The journal welcomes translational research, which involves the application of basic scientific findings to human health and clinical practice.
Clinical investigations: Cancer Cell is interested in publishing clinical investigations that contribute to establishing new paradigms in the treatment, diagnosis, or prevention of cancers.
Insights into cancer biology: The journal values clinical investigations that provide important insights into cancer biology beyond what has been revealed by preclinical studies.
Mechanism-based proof-of-principle studies: Cancer Cell encourages the publication of mechanism-based proof-of-principle clinical studies, which demonstrate the feasibility of a specific therapeutic approach or diagnostic test.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


