CHI3L3+未成熟中性粒细胞抑制骨转移的抗肿瘤免疫并阻碍免疫检查点阻断治疗
IF 44.5
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
摘要
骨转移瘤仍然无法治愈,对免疫检查点阻断(ICB)治疗反应不佳。中性粒细胞成熟对癌症的影响,特别是对包括骨转移在内的难治性肿瘤的影响,目前还不清楚。在这里,我们观察到未成熟中性粒细胞在小鼠模型和癌症患者的骨转移微环境中占主导地位。Dickkopf1 (DKK1)在中性粒细胞中诱导一种不成熟的功能状态,中性粒细胞表现出强大的免疫抑制能力,可以抑制CD8+ T细胞的抗肿瘤反应。在机制上,DKK1-CKAP4-STAT6信号通路驱动CHI3L3的表达,这是骨转移中未成熟中性粒细胞介导的免疫抑制所必需的。此外,在多发性骨转移小鼠模型中,阻断DKK1可促进中性粒细胞成熟,改善免疫微环境,诱导肿瘤缩小,并增强ICB治疗反应。这些发现揭示了未成熟中性粒细胞在骨转移中的关键作用,并提出了调节中性粒细胞以改善癌症免疫治疗的潜在策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
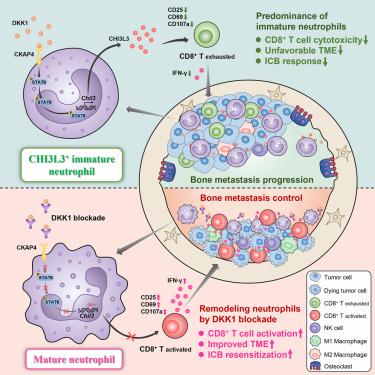
CHI3L3+ immature neutrophils inhibit anti-tumor immunity and impede immune checkpoint blockade therapy in bone metastases
Bone metastases remain incurable and respond poorly to immune checkpoint blockade (ICB) therapy. The impact of neutrophil maturation in cancer, particularly in refractory tumors including bone metastases, is not well understood. Here, we observed the predominance of immature neutrophils in the bone metastasis microenvironment of both mouse models and cancer patients. Dickkopf1 (DKK1) induces an immature-like functional state in neutrophils, which exhibit robust immunosuppressive capabilities to inhibit anti-tumor response of CD8+ T cells. Mechanistically, the DKK1-CKAP4-STAT6 signaling pathway drives CHI3L3 expression, which is necessary for the immune suppression mediated by immature neutrophils in bone metastases. Moreover, blocking DKK1 promotes neutrophil maturation to improve the immune microenvironment, induces tumor shrinkage, and enhances ICB therapy response in multiple bone metastasis mouse models. These findings uncover a critical role for immature neutrophils in bone metastases and suggest a potential strategy for modulating neutrophils to improve cancer immunotherapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer Cell
医学-肿瘤学
CiteScore
55.20
自引率
1.20%
发文量
179
审稿时长
4-8 weeks
期刊介绍:
Cancer Cell is a journal that focuses on promoting major advances in cancer research and oncology. The primary criteria for considering manuscripts are as follows:
Major advances: Manuscripts should provide significant advancements in answering important questions related to naturally occurring cancers.
Translational research: The journal welcomes translational research, which involves the application of basic scientific findings to human health and clinical practice.
Clinical investigations: Cancer Cell is interested in publishing clinical investigations that contribute to establishing new paradigms in the treatment, diagnosis, or prevention of cancers.
Insights into cancer biology: The journal values clinical investigations that provide important insights into cancer biology beyond what has been revealed by preclinical studies.
Mechanism-based proof-of-principle studies: Cancer Cell encourages the publication of mechanism-based proof-of-principle clinical studies, which demonstrate the feasibility of a specific therapeutic approach or diagnostic test.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


