一种泛kras抑制剂及其衍生的降解剂在kras驱动的癌症中具有多方面的抗肿瘤功效
IF 44.5
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
摘要
KRAS仍然是一个具有挑战性的治疗靶点,目前可用的有效抑制剂有限。在这里,我们报告了MCB-294的发现,这是一种有效的双态泛KRAS抑制剂,能够结合活性(gtp结合)和非活性(gdp结合)形式的KRAS。MCB-294通过水介导的氢键网络与开关- ii口袋接合,并选择性地抑制NRAS和HRAS之上的KRAS。在多种临床前模型中,它有效抑制致癌KRAS信号,抑制KRAS依赖性癌细胞和患者来源的类器官的生长,并减缓肿瘤进展。与非活性状态的选择性泛kras抑制剂Bl-2865和KRASG12D抑制剂MRTX1133相比,MCB-294也显示出更高的活性。在MCB-294作为pan-KRAS目标战斗部的基础上,我们进一步开发了MCB-36,一种von Hippel-Lindau (VHL)招募的pan-KRAS降解剂,可诱导持续的KRAS降解。值得注意的是,MCB-294和MCB-36都能有效抑制KRASG12C抑制剂耐药的癌细胞,重塑肿瘤免疫微环境。这些发现突出了广泛靶向kras驱动肿瘤和克服耐药性的有希望的治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
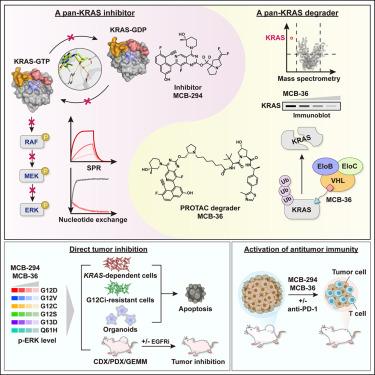
A pan-KRAS inhibitor and its derived degrader elicit multifaceted anti-tumor efficacy in KRAS-driven cancers
KRAS remains a challenging therapeutic target with limited effective inhibitors currently available. Here, we report the discovery of MCB-294, a potent dual-state pan-KRAS inhibitor capable of binding both the active (GTP-bound) and inactive (GDP-bound) forms of KRAS. MCB-294 engages the switch-II pocket through a water-mediated hydrogen-bond network and selectively inhibits KRAS over NRAS and HRAS. It effectively suppresses oncogenic KRAS signaling, inhibits the growth of KRAS-dependent cancer cells and patient-derived organoids, and reduces tumor progression in multiple preclinical models. MCB-294 also demonstrates superior activity compared to the inactive-state selective pan-KRAS inhibitor Bl-2865 and the KRASG12D inhibitor MRTX1133. Building upon MCB-294 as a pan-KRAS-targeting warhead, we further develop MCB-36, a von Hippel-Lindau (VHL)-recruiting pan-KRAS degrader that induces sustained KRAS degradation. Notably, both MCB-294 and MCB-36 effectively suppress KRASG12C inhibitor-resistant cancer cells and remodel the tumor immune microenvironment. These findings highlight a promising therapeutic strategy for broadly targeting KRAS-driven tumors and overcoming drug resistance.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer Cell
医学-肿瘤学
CiteScore
55.20
自引率
1.20%
发文量
179
审稿时长
4-8 weeks
期刊介绍:
Cancer Cell is a journal that focuses on promoting major advances in cancer research and oncology. The primary criteria for considering manuscripts are as follows:
Major advances: Manuscripts should provide significant advancements in answering important questions related to naturally occurring cancers.
Translational research: The journal welcomes translational research, which involves the application of basic scientific findings to human health and clinical practice.
Clinical investigations: Cancer Cell is interested in publishing clinical investigations that contribute to establishing new paradigms in the treatment, diagnosis, or prevention of cancers.
Insights into cancer biology: The journal values clinical investigations that provide important insights into cancer biology beyond what has been revealed by preclinical studies.
Mechanism-based proof-of-principle studies: Cancer Cell encourages the publication of mechanism-based proof-of-principle clinical studies, which demonstrate the feasibility of a specific therapeutic approach or diagnostic test.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


