MUC1-C与EBNA1的自调节复合体是潜在的eb病毒相关胃癌进展的原因。
IF 7.3
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
潜伏的eb病毒(EBV)感染促进来自b淋巴细胞和上皮细胞的癌症,其机制在很大程度上仍不清楚。ebv编码核抗原1 (EBNA1)在ebv相关癌症中均匀表达;然而,EBNA1如何促进癌症进展尚不清楚。在哺乳动物中,MUC1基因的进化是为了保护屏障组织免受病毒感染。我们报道,MUC1在ebv相关胃癌(EBVaGCs)中上调。我们的研究结果表明,EBNA1和致癌MUC1-C亚基形成了一个自动调节复合体,控制EBNA1、MUC1-C和宿主细胞基因的表达。EBNA1利用MUC1-C (i)诱导DNA甲基转移酶(DNMT)表达和DNA甲基化,(ii)抑制编码p21的CDKN1A以促进增殖,以及(iii)上调survivin以赋予生存。MUC1-C也被用于EBNA1在染色质中的定位、EBV潜伏期基因的表达和裂解基因的抑制。靶向MUC1-C从而诱导EBV潜伏期转换为裂解期的激活。我们进一步证明MUC1-C在EBVaGC干细胞(CSC)状态中是必需的,如NOTCH干细胞基因的调控和自我更新能力。这些发现以及EBV阳性对GCs患者生存无显著影响的证明表明,EBNA1利用MUC1-C维持EBV潜伏期,慢性EBV感染时MUC1-C的延长激活促进EBVaGC恶性进展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
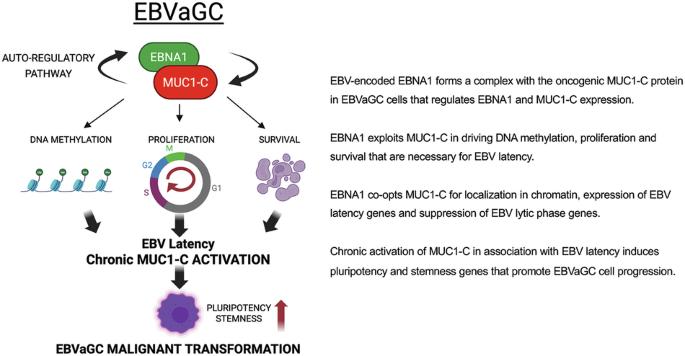
MUC1-C auto-regulatory complex with EBNA1 is responsible for latent Epstein-Barr virus-associated gastric cancer progression
Latent Epstein-Barr Virus (EBV) infection promotes cancers derived from B-lymphocytes and epithelial cells by mechanisms that largely remain unclear. EBV-encoded nuclear antigen 1 (EBNA1) is uniformly expressed in EBV-associated cancers; however, how EBNA1 contributes to cancer progression is not known. The MUC1 gene evolved in mammals to protect barrier tissues from viral infections. We report that MUC1 is upregulated in EBV-associated gastric cancers (EBVaGCs). Our results demonstrate that EBNA1 and the oncogenic MUC1-C subunit form an auto-regulatory complex that controls expression of EBNA1, MUC1-C and host cellular genes. EBNA1 appropriates MUC1-C to (i) induce DNA methyltransferase (DNMT) expression and DNA methylation, (ii) suppress CDKN1A encoding p21 to promote proliferation, and (iii) upregulate survivin to confer survival. MUC1-C is also co-opted for localization of EBNA1 in chromatin, expression of EBV latency genes and suppression of lytic genes. Targeting MUC1-C thereby induces the switch of EBV latency to activation of the lytic phase. We further demonstrate that MUC1-C is necessary for EBVaGC stem cell (CSC) state as evidenced by regulation of NOTCH stemness genes and self-renewal capacity. These findings and the demonstration that EBV positivity has no significant effect on survival of patients with GCs indicate that EBNA1 exploits MUC1-C to maintain EBV latency and that prolonged activation of MUC1-C in response to chronic EBV infection promotes EBVaGC malignant progression.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


