萘醌衍生物作为非小细胞肺癌铁下垂诱变剂的支架跳跃优化。
IF 2.2
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
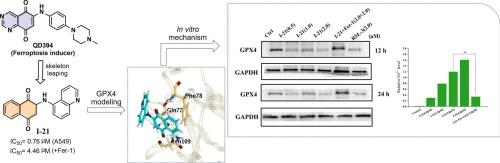
铁下垂是一种铁依赖的程序性细胞死亡途径,已成为一种有希望的癌症治疗靶点。本文以先导化合物QD394为先导,通过跳架优化设计了一系列萘醌衍生物,合成并评价了其对5种癌细胞的抗增殖活性,并描述了其构效关系(SAR)。其中,I-21活性最强,对A549细胞株的IC50 = 0.76 μM具有显著的体外抗癌作用。这种细胞毒性作用可以被铁抑制素-1抵消,这表明I-21可能作为铁凋亡诱导剂。机制研究表明,I-21通过消耗谷胱甘肽(GSH),升高活性氧(ROS)和丙二醛(MDA),下调谷胱甘肽过氧化物酶4(GPX4)表达,在A549细胞株中引发铁凋亡。此外,I-21将细胞周期阻滞在G2/M期,并抑制A549细胞株的迁移。本研究首次证实萘醌类衍生物可作为铁下垂诱导剂,为非小细胞肺癌的治疗提供了新的先导化合物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Scaffold-leaping optimization of naphthoquinone derivatives as ferroptosis inducer against non-small lung cancer
Ferroptosis, an iron-dependent programmed cell death pathway, has emerged as a promising therapeutic target for cancer. Herein, a series of naphthoquinone derivatives were designed via scaffold-leaping optimization from lead compound QD394, synthesized and assessed for their anti-proliferation activity against five cancer cell lines, and the structure-activity relationship (SAR) were described. Of these compounds, I-21 was identified as most active, demonstrating significant anticancer efficacy in vitro (IC50 = 0.76 μM against A549 cell lines). This cytotoxic effect can be counteracted by ferrostatin-1, a ferroptosis inhibitor, indicating that I-21 may acts as a ferroptosis inducer. Mechanistic studies revealed that I-21 triggered ferroptosis by depleting glutathione (GSH), elevating reactive oxygen species (ROS) and malondialdehyde (MDA), and downregulating glutathione peroxidase 4(GPX4) expression in A549 cell lines. Furthermore, I-21 arrested the cell cycle at the G2/M phase and inhibited the migration of A549 cell lines. This study provided the first evidence of naphthoquinone derivatives as ferroptosis inducers, offering a novel leading compound for the non-small cell lung cancer treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.70
自引率
3.70%
发文量
463
审稿时长
27 days
期刊介绍:
Bioorganic & Medicinal Chemistry Letters presents preliminary experimental or theoretical research results of outstanding significance and timeliness on all aspects of science at the interface of chemistry and biology and on major advances in drug design and development. The journal publishes articles in the form of communications reporting experimental or theoretical results of special interest, and strives to provide maximum dissemination to a large, international audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


