肿瘤浸润性伤害感受器神经元促进免疫抑制
IF 6.6
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
肿瘤释放的小细胞外囊泡(sev)将伤害受体神经元招募到肿瘤床。本研究发现,在小鼠头颈癌和黑色素瘤模型中,切除这些神经元可减少髓源性抑制细胞(MDSCs)的浸润。此外,在缺乏伤害感受器神经元的小鼠中,sev缺陷肿瘤不能发展。我们研究了头颈部鳞状细胞癌(HNSCC)和黑色素瘤中肿瘤浸润性伤害感受器与免疫细胞的相互作用。暴露于癌源性sev后,小鼠背根神经节(DRG)神经元分泌P物质、IL-6和损伤相关神经元标志物的量增加。患者源性sev使DRG神经元对辣椒素敏感,这意味着伤害感受器的反应性增强。此外,sev培养的伤害感受器诱导CD8+ T细胞的免疫抑制状态。用神经元和癌细胞共培养的条件培养基孵育,增加了MDSCs标志物的表达和原代骨髓细胞的抑制功能,而神经元条件培养基和癌症sev的结合促进了T细胞上检查点受体的表达。综上所述,这些发现揭示了伤害受体神经元促进CD8+ T细胞耗竭,并促进MDSC向HNSCC和黑色素瘤的浸润。因此,靶向伤害感受器可能提供一种策略来破坏癌症中有害的神经免疫串扰并增强抗肿瘤免疫。本文章由计算机程序翻译,如有差异,请以英文原文为准。
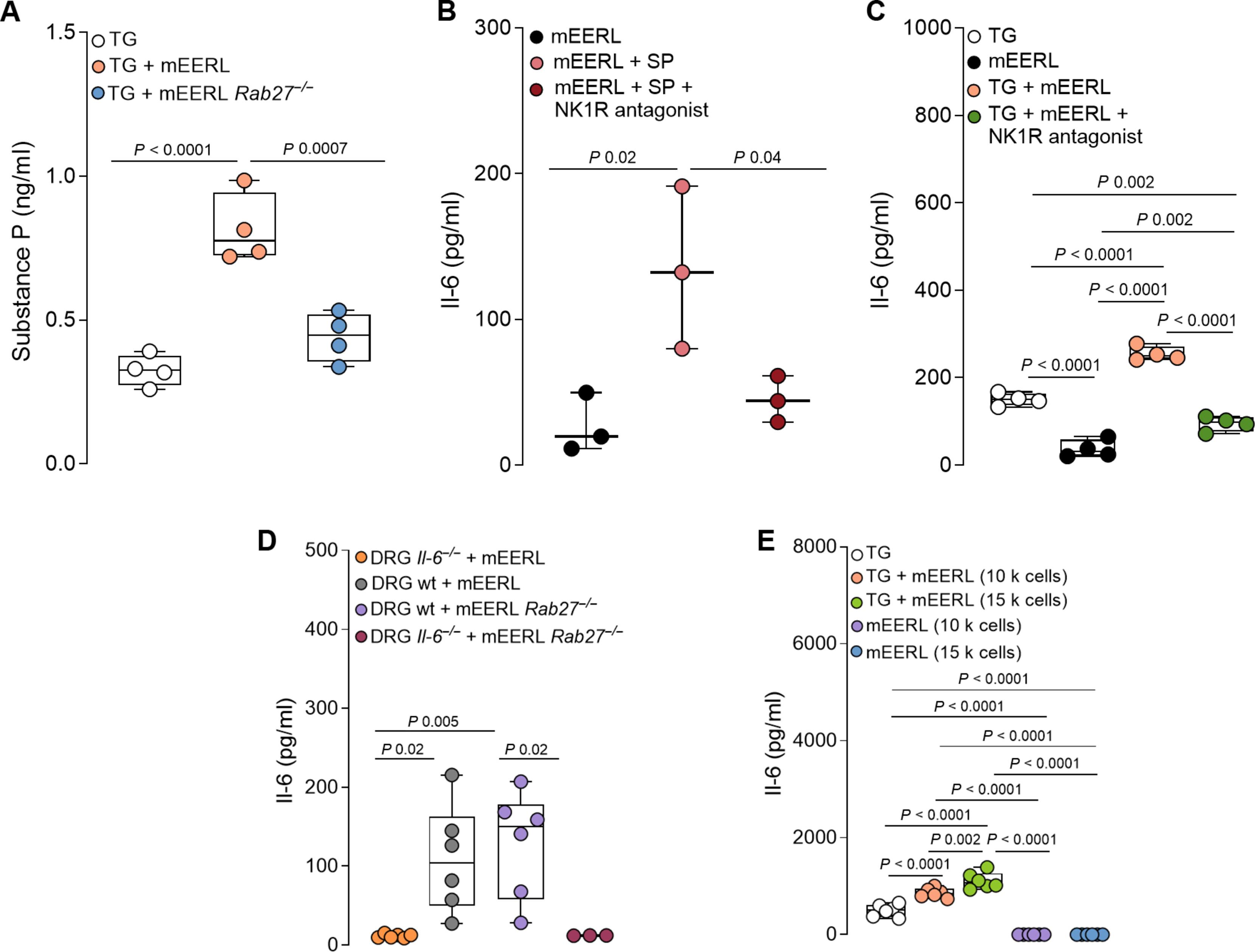
Tumor-infiltrating nociceptor neurons promote immunosuppression
Small extracellular vesicles (sEVs) released from tumors recruit nociceptor neurons to the tumor bed. Here, we found that ablating these neurons in mouse models of head and neck carcinoma and melanoma reduced the infiltration of myeloid-derived suppressor cells (MDSCs). Moreover, sEV-deficient tumors failed to develop in mice lacking nociceptor neurons. We investigated the interplay between tumor-infiltrating nociceptors and immune cells in head and neck squamous cell carcinoma (HNSCC) and melanoma. Upon exposure to cancer-derived sEVs, mouse dorsal root ganglion (DRG) neurons secreted increased amounts of substance P, IL-6, and injury-associated neuronal markers. Patient-derived sEVs sensitized DRG neurons to capsaicin, implying enhanced nociceptor responsiveness. Furthermore, nociceptors cultured with sEVs induced an immunosuppressed state in CD8+ T cells. Incubation with conditioned medium from cocultures of neurons and cancer cells resulted in increased expression of markers of MDSCs and suppressive function in primary bone marrow cells, and the combination of neuron-conditioned medium and cancer sEVs promoted checkpoint receptor expression on T cells. Together, these findings reveal that nociceptor neurons facilitate CD8+ T cell exhaustion and bolster MDSC infiltration into HNSCC and melanoma. Consequently, targeting nociceptors may provide a strategy to disrupt detrimental neuroimmune cross-talk in cancer and potentiate antitumor immunity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Signaling
BIOCHEMISTRY & MOLECULAR BIOLOGY-CELL BIOLOGY
CiteScore
9.50
自引率
0.00%
发文量
148
审稿时长
3-8 weeks
期刊介绍:
"Science Signaling" is a reputable, peer-reviewed journal dedicated to the exploration of cell communication mechanisms, offering a comprehensive view of the intricate processes that govern cellular regulation. This journal, published weekly online by the American Association for the Advancement of Science (AAAS), is a go-to resource for the latest research in cell signaling and its various facets.
The journal's scope encompasses a broad range of topics, including the study of signaling networks, synthetic biology, systems biology, and the application of these findings in drug discovery. It also delves into the computational and modeling aspects of regulatory pathways, providing insights into how cells communicate and respond to their environment.
In addition to publishing full-length articles that report on groundbreaking research, "Science Signaling" also features reviews that synthesize current knowledge in the field, focus articles that highlight specific areas of interest, and editor-written highlights that draw attention to particularly significant studies. This mix of content ensures that the journal serves as a valuable resource for both researchers and professionals looking to stay abreast of the latest advancements in cell communication science.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


