MePhTIM大环负载的金属有机铁(III)配合物:π-酸度调节的尝试
IF 2.1
3区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
本文报道了苯乙酰基配合物反式-[Fe(III)(MePhTIM)(C2Ph)2]+(2)、σ−苯基配合物反式-[Fe(III)(MePhTIM)(Ph)2]+(3)及其前体反式-[Fe(III)(MePhTIM)Cl2]+(1)的合成和表征,它们都由四亚胺大环MePhTIM(2,9,-二甲基-3,10-二苯基-1,4,8,11-四氮杂环十四十四-1,3,8,10-四烯)支撑。利用质谱法、电子吸收光谱法、循环和微分脉冲伏安法等技术对这些新型配合物进行了研究。利用单晶x射线衍射分析确定了化合物1、2和3的分子结构,并利用密度泛函理论(DFT)计算分析了它们的电子结构(基态和激发态)。该配合物表现出丰富的氧化还原特性,包括可逆FeIII/II还原和MePhTIM还原。TD-DFT计算表明,引入基于mephtim的π(苯基)轨道,即π(苯基宏),产生了新的1和2的电荷转移带。此外,电化学和DFT结果表明,与类似的TIM(2,3,9,10-四甲基-1,4,8,11-四氮杂环十四十四-1,3,8,10-四烯)相比,所有配合物的Fe dπ轨道都具有更大的稳定性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
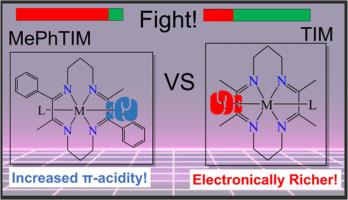
Organometallic iron(III) complexes supported by MePhTIM macrocycle: An attempt of π-acidity tuning
Reported herein are the syntheses and characterization of the phenylacetylide complex trans-[Fe(III)(MePhTIM)(C2Ph)2]+(2), σ−phenyl complex trans-[Fe(III)(MePhTIM)(Ph)2]+(3), and their precursor trans-[Fe(III)(MePhTIM)Cl2]+(1), all of which are supported by the tetra-imine macrocycle MePhTIM (2,9,-dimethyl-3,10-diphenyl-1,4,8,11-tetraazacyclotetradeca-1,3,8,10-tetraene). All the new complexes were investigated using mass spectrometry, electronic absorption spectroscopy and cyclic and differential pulse voltammetry techniques. The molecular structures of compounds 1, 2, and 3 were established using single-crystal X-ray diffraction analysis, and their electronic structures (both ground and excited state) analyzed using density functional theory (DFT) calculations. The complexes display rich redox characteristics including a reversible FeIII/II reduction and a MePhTIM reduction. TD-DFT calculations show that the introduction of MePhTIM-based π(phenyl) orbitals, namely π(phenylmacro), yields new charge transfer bands for 1 and 2. Furthermore, a greater stabilization of the Fe dπ orbitals for all complexes in comparison to their analogous TIM (2,3,9,10-tetramethyl-1,4,8,11-tetraazacyclotetradeca-1,3,8,10-tetraene) counterparts is evident from electrochemistry and DFT results.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organometallic Chemistry
化学-无机化学与核化学
CiteScore
4.40
自引率
8.70%
发文量
221
审稿时长
36 days
期刊介绍:
The Journal of Organometallic Chemistry targets original papers dealing with theoretical aspects, structural chemistry, synthesis, physical and chemical properties (including reaction mechanisms), and practical applications of organometallic compounds.
Organometallic compounds are defined as compounds that contain metal - carbon bonds. The term metal includes all alkali and alkaline earth metals, all transition metals and the lanthanides and actinides in the Periodic Table. Metalloids including the elements in Group 13 and the heavier members of the Groups 14 - 16 are also included. The term chemistry includes syntheses, characterizations and reaction chemistry of all such compounds. Research reports based on use of organometallic complexes in bioorganometallic chemistry, medicine, material sciences, homogeneous catalysis and energy conversion are also welcome.
The scope of the journal has been enlarged to encompass important research on organometallic complexes in bioorganometallic chemistry and material sciences, and of heavier main group elements in organometallic chemistry. The journal also publishes review articles, short communications and notes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


