从物理、化学(cDFT)和地质背景看长英质trans-斑岩矿床及其伴生矿石(Sn、Ta、Nb、伟晶岩)
IF 2.5
2区 地球科学
Q2 GEOCHEMISTRY & GEOPHYSICS
引用次数: 0
摘要
一些贱金属(Cu、Mo、W、Sn)是在斑岩矿床岩浆期从硅酸盐熔体中提取出来的。金属品位从一些ppb或ppm增加到百分比水平。常见的情况是俯冲带以上的斑岩型(Cu、Mo)。介绍了大陆板块内由克拉通间大规模剪切作用形成的斑岩型(锡)矿床。全球成矿过程具有岩浆生成、侵位和非混相(MIP)形成的三重作用。在略高于800°C的熔化过程中,金属首先从地壳中分离出来。在岩浆上升的过程中,MIP保持在临界点(731°C)以上,并以化学方式吸引金属,比熔化更快地运送金属。金属从熔体中分离发生在亚临界状态。它依赖于化学势对比(化学分配),由它们的微分运动(粘度、密度)增强。化学扩散与平流负竞争,产生富集。随着冷却,熔体分裂成两个相,富含硅酸盐和以水为主,两者都不能与熔体和基体混溶。金属的扩散系数、熔体和基质粘度、盖层渗透率以及流体的数量都在参数化公式中进行了研究。它排除了通过扩散产生的温和的化学分化。金属(Sn, Nb, Ta)偏析成伟晶岩和辉绿岩是成矿的最终产物。基于岩石(花岗岩、伟晶岩、灰岩)整体组成的模型显示出不足,因为它们没有包含足够数量的流体。物理参数也用化学描述符(化学势、硬度)括起来,这些描述符支配熔体和基体的演化。物理环境和化学吸引力的综合研究为岩浆环境下的这种成矿提供了新的见解。275 w。本文章由计算机程序翻译,如有差异,请以英文原文为准。
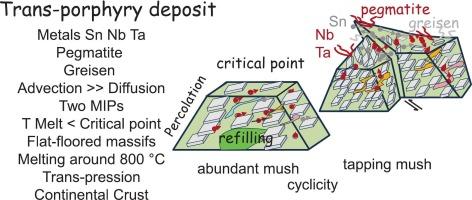
Felsic trans-porphyry deposits and associated ore (Sn, Ta, Nb, pegmatites) viewed from physics, chemistry (cDFT) and geologic contexts
Some base metals (Cu, Mo, W, Sn) are extracted from silicate melts at the magmatic stage in porphyry deposits. Metal grade increases from some ppb or ppm up to percent levels. The common case is the porphyry type (Cu, Mo) above subduction zones. We introduce the trans-porphyry type deposits (Sn) within continental plates, resulting from large-scale shearing between cratons. The global process for ore generation has a three-fold effect, from magma generation, emplacement, and formation of a immiscible phase (MIP). Metals first segregate from the crust during melting slightly above 800 °C. During magma ascent, the MIP remains above the critical point (731 °C), and chemically attracts metals, transporting them faster than the melt. Metals segregation from the melt occurs in a subcritical state. It relies on the chemical potential contrast (chemical partitioning), enhanced by their differential motion (viscosity, density). Chemical diffusion negatively competes with advection, yielding enrichment. With cooling, the melt splits into two phases, silicate-rich and aqueous-dominated, both are immiscible with the melt and the matrix. Metals diffusivity, melt and matrix viscosity, cap rock permeability, and the amount of fluids are investigated in a parametric formulation. It rules out a gentle chemical differentiation though diffusion. Metals segregation (Sn, Nb, Ta) into pegmatite and greisen are the ultimate products of ore formation. Models based on the bulk composition of rocks (granite, pegmatites, greisens) reveal inadequate since they do not incorporate fluids in enough quantity. The physical parameters are also bracketed by the chemical descriptors (chemical potential, hardness) that rule melt and matrix evolution. The combined investigation of the physical context and chemical attractivity provides new insights into such ore formation in a magmatic context. 275 w.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Lithos
地学-地球化学与地球物理
CiteScore
6.80
自引率
11.40%
发文量
286
审稿时长
3.5 months
期刊介绍:
Lithos publishes original research papers on the petrology, geochemistry and petrogenesis of igneous and metamorphic rocks. Papers on mineralogy/mineral physics related to petrology and petrogenetic problems are also welcomed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


