香豆素锌衍生物对催产素受体的调节:来曲唑诱导的斑马鱼PCOS模型中缓解焦虑和促进卵泡生成的机制途径
IF 3.1
4区 生物学
Q2 BIOLOGY
引用次数: 0
摘要
多囊卵巢综合征(PCOS)是一种常见的女性内分泌疾病,以胰岛素抵抗和情绪障碍为特征。传统疗法的治疗潜力往往受到副作用的限制,因此需要新的干预措施。本研究研究了新合成的硫代氨基脲酮香豆素锌配合物的疗效,并在来曲唑诱导的PCOS斑马鱼体内模型中评估了TSCO6-Zn (T1)和TSCO13-Zn (T2)。通过Pechmann缩合生成7-羟基-4-甲基香豆素,然后与锌配位。通过BindingDB预测化合物的靶点,并通过分子对接确认与pcos相关蛋白的相互作用。对暴露于T1和T2(高达150 µM)的斑马鱼胚胎进行体内毒性评估,检查行为分析、体重、脂质谱、GSI(%)和卵泡发生。此外,对20β-hsd、cyp19a1a、dennd1a、tox3、oxtr、mTOR、pik3cd、drd2a进行了HPLC睾丸激素定量和qPCR基因表达分析。T1和T2显著减少焦虑,降低睾酮,促进卵泡成熟,高达50 µM无毒性观察。对接研究表明T1和T2对drd2a、oxtr、mTOR和pik3cd等关键代谢和神经行为靶点具有高亲和力。在来曲唑诱导的多囊卵巢综合征中,斑马鱼的体重、脂质谱、氧化应激标志物以及参与类固醇生成和代谢途径的规范化基因表达均有显著改善。在斑马鱼模型中,T1和T2可有效缓解PCOS相关的代谢和神经行为障碍,提示其作为综合治疗剂的潜力。他们的多靶点方法可以为PCOS的高级治疗策略提供基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
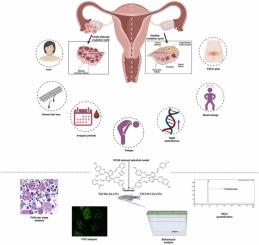
Regulation of oxytocin receptor by zinc coumarin derivatives: a mechanistic approach to alleviate anxiety and enhance folliculogenesis in letrozole-induced PCOS in zebrafish model
Polycystic ovary syndrome (PCOS) is a common endocrine disorder in women, characterized by insulin resistance and mood disturbances. The therapeutic potential of traditional treatments is often limited by side effects, highlighting the need for novel interventions. This study investigated the efficacy of newly synthesized thiosemicarbazone coumarin zinc complexes, TSCO6-Zn (T1) and TSCO13-Zn (T2) were assessed in a letrozole-induced PCOS in-vivo zebrafish model. Synthesis involved Pechmann condensation to form 7-hydroxy-4-methylcoumarin, followed by coordination with zinc. The compounds’ targets were predicted via BindingDB, with molecular docking confirming interactions with PCOS-related proteins. In vivo toxicity was assessed in zebrafish embryos exposed to T1 and T2 (up to 150 µM), examining behavioral assays, body weight, lipid profile, GSI (%) and folliculogenesis. In addition to that, HPLC testosterone quantification and qPCR for gene expression analysis were employed for 20β-hsd, cyp19a1a, dennd1a, tox3, oxtr, mTOR, pik3cd, and drd2a. T1 and T2 markedly reduced anxiety, lowered testosterone, and enhanced follicular maturation, with no toxicity observed up to 50 µM. Docking studies demonstrated a high affinity of T1 and T2 for key metabolic and neurobehavioral targets such as drd2a, oxtr, mTOR, and pik3cd. Significant improvements were noted in body weight, lipid profiles, oxidative stress markers, and normalized gene expressions involved in steroidogenesis and metabolic pathways in letrozole-induced PCOS in the zebrafish. T1 and T2 effectively mitigate metabolic and neurobehavioral disturbances associated with PCOS in the zebrafish model, suggesting their potential as comprehensive therapeutic agents. Their multi-target approach could provide a basis for advanced PCOS treatment strategies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Computational Biology and Chemistry
生物-计算机:跨学科应用
CiteScore
6.10
自引率
3.20%
发文量
142
审稿时长
24 days
期刊介绍:
Computational Biology and Chemistry publishes original research papers and review articles in all areas of computational life sciences. High quality research contributions with a major computational component in the areas of nucleic acid and protein sequence research, molecular evolution, molecular genetics (functional genomics and proteomics), theory and practice of either biology-specific or chemical-biology-specific modeling, and structural biology of nucleic acids and proteins are particularly welcome. Exceptionally high quality research work in bioinformatics, systems biology, ecology, computational pharmacology, metabolism, biomedical engineering, epidemiology, and statistical genetics will also be considered.
Given their inherent uncertainty, protein modeling and molecular docking studies should be thoroughly validated. In the absence of experimental results for validation, the use of molecular dynamics simulations along with detailed free energy calculations, for example, should be used as complementary techniques to support the major conclusions. Submissions of premature modeling exercises without additional biological insights will not be considered.
Review articles will generally be commissioned by the editors and should not be submitted to the journal without explicit invitation. However prospective authors are welcome to send a brief (one to three pages) synopsis, which will be evaluated by the editors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


