镍催化环氨基磺酸亚胺不对称加氢制手性环氨基磺酸对映选择性的DFT研究
IF 3
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
本研究利用手性(S,S)-Ph-BPE配体,通过DFT计算(ωB97XD/ 6-311 + G(d,p), SMD(TFE) // ωB97XD/6-31G(d,p), SMD(TFE), SDD for Ni),阐明了Ni催化环氨基磺酸亚胺不对称加氢生成手性环氨基磺酸盐的对映选择性的来源。DFT结果表明,[Ni]-H在环磺胺亚胺的CN键上的加成是决定对映选择性的步骤。期望的s产物的高对映选择性归因于s途径中有利的CH-π吸引相互作用,而以孤对π排斥为特征的r途径能量上不太有利,导致较低的对映选择性。此外,利用能量跨度模型和过渡态理论计算的对映体过量(ee)超过99%有利于s对映体。尽管如此,dft预测的%ee值定性地与实验观察到的94%的ee对映体选择性一致。此外,将底物的R取代基由苯基修饰为甲基,可显著降低ΔΔG‡,从苯基的3.9 kcal/mol降至甲基的2.2 kcal/mol。这一趋势与实验结果一致。这些发现为开发环氨基磺酸亚胺对映选择性加氢的高级手性催化剂奠定了基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
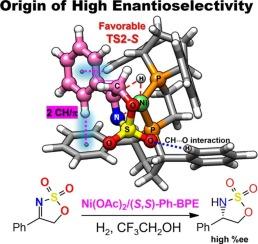
DFT study of the origin of enantioselectivity of Ni-catalyzed asymmetric hydrogenation of cyclic sulfamidate imine to chiral cyclic sulfamidates
In this study, we elucidate the origin of enantioselectivity in Ni-catalyzed asymmetric hydrogenation of cyclic sulfamidate imines to chiral cyclic sulfamidates using the chiral (S,S)-Ph-BPE ligand, based on DFT calculations (ωB97XD/6–311 + G(d,p), SMD(TFE) // ωB97XD/6-31G(d,p), SMD(TFE), SDD for Ni). The DFT results reveal that the addition of [Ni]-H to the C![]() N bond of the cyclic sulfamidate imine is the enantioselectivity-determining step. The high enantioselectivity of the desired S-product is attributed to favorable CH-π attractive interactions in the S-pathway, whereas the R-pathway, characterized by lone-pair-π repulsion, is energetically less favorable, leading to lower enantioselectivity. Furthermore, the enantiomeric excess (ee), calculated using the Energetic Span Model and Transition State Theory, exceeds 99 % in favor of the S-enantiomer. Despite this, the DFT-predicted %ee values qualitatively align with the experimentally observed enantioselectivity of ee >94 %. Additionally, modifying the R substituent of the substrate from phenyl to methyl resulted in a significant decrease in ΔΔG‡, from 3.9 kcal/mol for the phenyl group to 2.2 kcal/mol for the methyl group. This trend aligns with experimental results. These findings lay the groundwork for the development of advanced chiral catalysts for the enantioselective hydrogenation of cyclic sulfamidate imine substrates.
N bond of the cyclic sulfamidate imine is the enantioselectivity-determining step. The high enantioselectivity of the desired S-product is attributed to favorable CH-π attractive interactions in the S-pathway, whereas the R-pathway, characterized by lone-pair-π repulsion, is energetically less favorable, leading to lower enantioselectivity. Furthermore, the enantiomeric excess (ee), calculated using the Energetic Span Model and Transition State Theory, exceeds 99 % in favor of the S-enantiomer. Despite this, the DFT-predicted %ee values qualitatively align with the experimentally observed enantioselectivity of ee >94 %. Additionally, modifying the R substituent of the substrate from phenyl to methyl resulted in a significant decrease in ΔΔG‡, from 3.9 kcal/mol for the phenyl group to 2.2 kcal/mol for the methyl group. This trend aligns with experimental results. These findings lay the groundwork for the development of advanced chiral catalysts for the enantioselective hydrogenation of cyclic sulfamidate imine substrates.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Computational and Theoretical Chemistry
CHEMISTRY, PHYSICAL-
CiteScore
4.20
自引率
10.70%
发文量
331
审稿时长
31 days
期刊介绍:
Computational and Theoretical Chemistry publishes high quality, original reports of significance in computational and theoretical chemistry including those that deal with problems of structure, properties, energetics, weak interactions, reaction mechanisms, catalysis, and reaction rates involving atoms, molecules, clusters, surfaces, and bulk matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


