基于多齿希夫碱配体的两种新型Gd2和Gd4化合物:不同的晶体结构和磁制冷性能
IF 3.3
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
在溶剂热条件下成功合成了两个新的Gd(III)基化合物[Gd2(L1)2(Ac)2(ch30)2] (1) (HL1 = (E)- n′-(5-溴-2-羟基-3-甲氧基苄基)picolinohydrazide)和[Gd4(L2)4(μ2-OH)(μ2- ch30)3(ch30)4] (2) (HL2 = (E)- n′-(3,5-二溴-2-羟基苄基)picolinohydrazide)。化合物1为双核结构,具有平行四边形Gd2O2核;化合物2为四核结构,具有菱形Gd4核。磁性研究表明,1- gd2和2-Gd4化合物在相邻的Gd(III)离子之间表现出弱的反铁磁耦合,并且具有较大的磁热效应,1- gd2的磁热效应为25.79 J kg−1 K−1 (ΔH = 70 kOe, 2.5 K), 2-Gd4的磁热效应为25.50 J kg−1 K−1 (ΔH = 70 kOe, 2.0 K)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
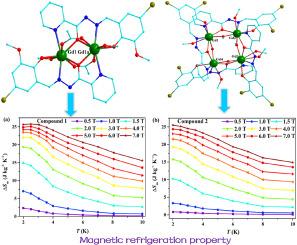
Two new Gd2 and Gd4 compounds based on multidentate Schiff-base ligands: Different crystal structures and magnetic refrigeration properties
Two new Gd(III)-based compounds [Gd2(L1)2(Ac)2(CH3O)2] (1) (HL1 = (E)-N'-(5-bromo-2-hydroxy-3-methoxybenzylidene)picolinohydrazide), and [Gd4(L2)4(μ2-OH)(μ2-CH3O)3(CH3O)4] (2) (HL2 = (E)-N'-(3,5-dibromo-2-hydroxybenzylidene)picolinohydrazide) were successfully synthesized under solvothermal conditions. Compound 1 is a binuclear structure and possesses a parallelogram Gd2O2 core, while compound 2 is a tetranuclear structure and contains a rhombic Gd4 core. Magnetic studies suggest that 1-Gd2 and 2-Gd4 compounds exhibits weak antiferromagnetic coupling between adjacent Gd(III) ions and have larger magnetocaloric effects of 25.79 J kg−1 K−1 for 1-Gd2 (ΔH = 70 kOe at 2.5 K) and 25.50 J kg−1 K−1 for 2-Gd4 (ΔH = 70 kOe at 2.0 K).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Solid State Sciences
化学-无机化学与核化学
CiteScore
6.60
自引率
2.90%
发文量
214
审稿时长
27 days
期刊介绍:
Solid State Sciences is the journal for researchers from the broad solid state chemistry and physics community. It publishes key articles on all aspects of solid state synthesis, structure-property relationships, theory and functionalities, in relation with experiments.
Key topics for stand-alone papers and special issues:
-Novel ways of synthesis, inorganic functional materials, including porous and glassy materials, hybrid organic-inorganic compounds and nanomaterials
-Physical properties, emphasizing but not limited to the electrical, magnetical and optical features
-Materials related to information technology and energy and environmental sciences.
The journal publishes feature articles from experts in the field upon invitation.
Solid State Sciences - your gateway to energy-related materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


