CRISPR/ cas9介导的PD-1衰减增强了人源化肝癌pdx模型中基于肿瘤浸润淋巴细胞的过继细胞治疗
IF 5
2区 医学
Q2 Medicine
引用次数: 0
摘要
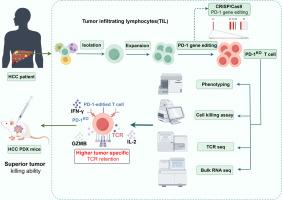
基于肿瘤浸润性淋巴细胞(TIL)的过继细胞疗法(ACT)由于能够有效控制多种类型实体瘤的疾病而成为一种很有前途的治疗方法。针对阴性免疫检查点程序性细胞死亡蛋白1 (PD-1)的抗体已广泛应用于肿瘤免疫治疗。我们假设在肝细胞癌(HCC) til衍生的T细胞中PD-1缺失会增强其抗肿瘤功效。方法采用scrispr / Cas9系统靶向肝癌til源性T细胞中的PDCD1。用流式细胞术分析其表型和功能变化。对pd -1编辑或未编辑的T细胞进行T细胞受体(TCR)测序和大量rna测序,以检查它们的差异。最后,我们证明了pd -1编辑或未编辑的T细胞在HCC患者来源的异种移植(PDX)模型中抑制肿瘤生长的能力。结果crispr /Cas9系统提供了有效且稳定的pd -1编辑效率。pd -1编辑的T细胞的表型、效应和记忆亚群保持了稳定性,同时它们在自体肿瘤细胞消除方面确实获得了更高的潜力。与pd -1编辑的T细胞相比,pd -1编辑的T细胞还与原发性HCC til的整体和肿瘤特异性TCR克隆型保持了更高水平的同源性。此外,在HCC PDX模型中,pd -1编辑的T细胞比未编辑的T细胞表现出更好的抗肿瘤反应。综上所述,我们的研究结果为基于基因修饰TIL的ACT的临床应用奠定了基础,为探索HCC和其他实体肿瘤的临床免疫治疗策略提供了新的视角。本文章由计算机程序翻译,如有差异,请以英文原文为准。
CRISPR/Cas9-mediated PD-1 attenuation enhances tumor infiltrating lymphocyte-based adoptive cellular therapy in humanized-PDX model of hepatocellular carcinoma
Background
Tumor-infiltrating lymphocyte (TIL)-based adoptive cellular therapy (ACT) has become a promising therapeutic approach due to its ability to effectively control disease in multiple types of solid tumor. Antibodies against the negative immune checkpoint programmed cell death protein 1 (PD-1) have been widely used in cancer immunotherapy. We hypothesized that PD-1 depletion in hepatocellular carcinoma (HCC) TIL-derived T cells would enhance their anti-tumor efficacy.
Methods
CRISPR/ Cas9 system was employed to target PDCD1 in HCC TIL-derived T cells. The phenotypic and functional changes were analyzed by flow cytometry. T-cell receptor (TCR) sequencing and bulk RNA-sequencing of PD-1-edited or non-edited T cells was conducted to examine their differences. Finally, we demonstrated the ability of PD-1-edited or non-edited T cells to inhibit tumor growth in HCC patient-derived xenograft (PDX) models.
Results
CRISPR/Cas9 system was demonstrated to provide an effective and stable PD-1-editing efficiency. The phenotypes, effector and memory subpopulations of the PD-1-edited T cells were found to have maintained stability, while they did acquire higher potential in terms of autologous tumor cell elimination. Compared to their counterpart, PD-1-edited T cells also retained a higher level of homology with the whole and tumor-specific TCR clonotypes of primary HCC TILs. Furthermore, PD-1-edited T cells exhibited a superior anti-tumor response compared with non-edited T cells in HCC PDX models.
Conclusion
Taken together, our results have provided a foundation for the clinical application of ACT based on genetically modified TIL, offering a new perspective on exploring clinical immunotherapy strategies for HCC, and potentially other solid tumors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Translational Oncology
ONCOLOGY-
CiteScore
8.40
自引率
2.00%
发文量
314
审稿时长
54 days
期刊介绍:
Translational Oncology publishes the results of novel research investigations which bridge the laboratory and clinical settings including risk assessment, cellular and molecular characterization, prevention, detection, diagnosis and treatment of human cancers with the overall goal of improving the clinical care of oncology patients. Translational Oncology will publish laboratory studies of novel therapeutic interventions as well as clinical trials which evaluate new treatment paradigms for cancer. Peer reviewed manuscript types include Original Reports, Reviews and Editorials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


