经典活化的巨噬细胞在一氧化氮的驱动下进行功能显著的核苷酸代谢重塑
IF 20.8
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
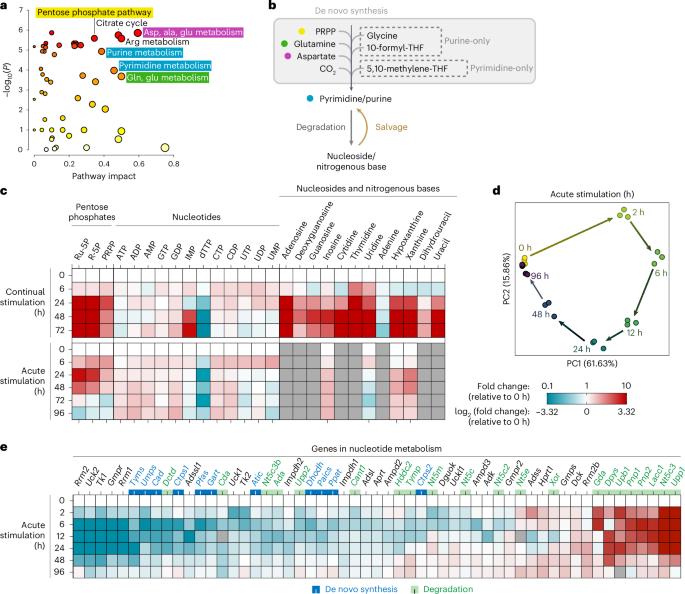
在免疫应答过程中,巨噬细胞特异性地重新编程其代谢以支持功能变化。在这里,我们发现核苷酸代谢是经典激活后最重要的重编程途径之一。具体来说,嘧啶的从头合成一直维持到单磷酸尿苷,但在三磷酸胞苷和单磷酸脱氧胸腺嘧啶合成时受阻;嘌呤的重新合成在最后一步被关闭(由AICAR转化酶/IMP环水解酶催化,ATIC),细胞转向增加嘌呤回收。核苷酸对含氮碱基的降解被上调,但嘌呤碱基的完全氧化(由黄嘌呤氧化还原酶(XOR)催化)被抑制,将通量转向回收。在机制上,一氧化氮被认为是核苷酸代谢的主要调节因子,同时驱动多种关键变化,包括Tyms的转录下调和ATIC和XOR的深度抑制。通过敲除或抑制Hgprt抑制嘌呤回收可以改变许多刺激诱导基因的表达,抑制巨噬细胞的迁移和吞噬,并增加细胞内寄生虫弓形虫的增殖。这些结果全面揭示了经典激活下巨噬细胞核苷酸代谢的动态重编程,阐明了这种重编程的调控机制和功能意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Classically activated macrophages undergo functionally significant nucleotide metabolism remodelling driven by nitric oxide
During an immune response, macrophages specifically reprogramme their metabolism to support functional changes. Here, we revealed that nucleotide metabolism is one of the most significantly reprogrammed pathways upon classical activation. Specifically, de novo synthesis of pyrimidines is maintained up to uridine monophosphate, but blocked at cytidine triphosphate and deoxythymidine monophosphate synthesis; de novo synthesis of purines is shut off at the last step (catalysed by AICAR transformylase/IMP cyclohydrolase, ATIC), and cells switch to increased purine salvage. Nucleotide degradation to nitrogenous bases is upregulated but complete oxidation of purine bases (catalysed by xanthine oxidoreductase, XOR) is inhibited, diverting flux into salvage. Mechanistically, nitric oxide was identified as a major regulator of nucleotide metabolism, simultaneously driving multiple key changes, including the transcriptional downregulation of Tyms and profound inhibition of ATIC and XOR. Inhibiting purine salvage using Hgprt knockout or inhibition alters the expression of many stimulation-induced genes, suppresses macrophage migration and phagocytosis, and increases the proliferation of the intracellular parasite Toxoplasma gondii. Together, these results thoroughly uncover the dynamic reprogramming of macrophage nucleotide metabolism upon classical activation and elucidate the regulatory mechanisms and functional significance of such reprogramming. John et al. show that upon classical activation, macrophages undergo nitric oxide-driven reprogramming of nucleotide metabolism, which affects immune functions and responses.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


