通过铜催化的硼化环化反应获得硼基甲基取代硅环丁烷的区域选择性和化学选择性。
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
硅环丁烷(SCBs)是构建复杂有机硅结构的关键合成物;然而,β功能化scb的合成途径仍未得到充分探索。在此,我们报道了一种新的Cu(I)催化的硼化环化策略,该策略能够从硅系链烯酰氯和双(pinacolato)二硼中进行区域和化学选择性合成β功能化scb。该方法为在温和条件下构建不同的硼基甲基取代硅环丁烷提供了一种可扩展的方法。安装的硼酸盐部分作为一个通用的合成手柄,用于访问复杂的含硅杂环,在有机硅化学中显示出重要的实用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
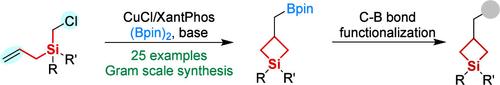
Regio- and Chemoselective Access to Borylmethyl-Substituted Silacyclobutanes via Copper-Catalyzed Borylative Cyclization
Silacyclobutanes (SCBs) are pivotal synthons for constructing complex organosilicon architectures; however, synthetic routes to β-functionalized SCBs remain underexplored. Herein we report a novel Cu(I)-catalyzed borylative cyclization strategy that enables regio- and chemoselective synthesis of β-functionalized SCBs from silicon-tethered alkenyl chlorides and bis(pinacolato)diboron. This method provides a scalable approach for the construction of diverse borylmethyl-substituted silacyclobutanes under mild conditions. The installed boronate moiety serves as a versatile synthetic handle for accessing complex silicon-containing heterocycles, demonstrating significant utility in organosilicon chemistry.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


