MCM基因的鉴定、表征和表达分析揭示了MCM基因在低ph胁迫下的调控作用。
IF 2.2
2区 生物学
Q4 BIOCHEMISTRY & MOLECULAR BIOLOGY
Comparative Biochemistry and Physiology D-Genomics & Proteomics
Pub Date : 2025-07-29
DOI:10.1016/j.cbd.2025.101591
引用次数: 0
摘要
Yesso扇贝(Patinopecten yessoensis)是一种经济上重要的双壳类物种,对海洋酸化具有高度敏感性。低ph胁迫导致的生长迟缓是叶梭扇贝养殖面临的重大挑战。然而,这种现象背后的分子机制尚不清楚。考虑到细胞增殖在生物生长中的关键作用,我们研究了叶红(P. yessoensis)的小染色体维持(MCM)家族,该家族是DNA复制起始和细胞周期调节的关键调节器。在本研究中,我们鉴定了9个MCM基因(PyMCM2-10)。这些pymcm具有高度保守的序列特征和典型的MCM结构域。系统发育分析表明,pymcm可分为9个不同的分支,强调了它们在物种同源物中的强进化保守性。时空表达谱显示,pymcm在所有发育阶段和成年组织中广泛表达,在旺盛的细胞增殖中表达水平特别高(例如,在多细胞阶段高达318 TPM,在性腺组织中高达202 TPM)。值得注意的是,PyMCM6在整个发育和组织中表现出一致的高表达(bbbb44 TPM),这表明在发育和组织维持中都起着关键的调节作用。在低ph胁迫下,pymcm的表达均不同程度下调,其中PyMCM5的表达下调最为显著(|log2FC|下调至3.5),而PyMCM8和PyMCM9则保持相对稳定。这种模式表明,扇贝降低DNA复制能力(由PyMCM2-7介导),但可能维持DNA修复功能(与pymcm2 /9稳定性相关),以减轻低ph诱导的损伤,这可能解释了细胞增殖的内在抑制。实时荧光定量PCR和原位杂交进一步证实,低ph胁迫抑制了PyMCMs的表达(p本文章由计算机程序翻译,如有差异,请以英文原文为准。
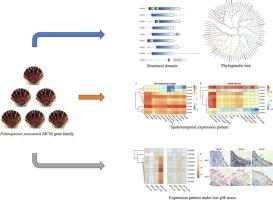
Identification, characterization, and expression analysis reveal regulatory roles of MCM genes in Patinopecten yessoensis under low-pH stress
The Yesso scallop (Patinopecten yessoensis), an ecomically important bivalve species, exhibits high susceptibility to ocean acidification. The growth retardation induced by low-pH stress poses a significant challenge for Yesso scallop aquaculture. However, the molecular mechanisms underlying this phenomenon are not well understood. Considering the pivotal role of cell proliferation in organism growth, we investigated the minichromosome maintenance (MCM) family in P. yessoensis, which are key regulators of DNA replication initiation and cell cycle regulation. In this study, we identified nine MCM genes (PyMCM2–10) in the P. yessoensis genome. These PyMCMs exhibit highly conserved sequence characteristics and typical MCM domains. Phylogenetic analysis showed that PyMCMs cluster into nine distinct clades, underscoring their strong evolutionary conservation across species homologs. Spatiotemporal expression profiling demonstrated widespread expressions of PyMCMs throughout all developmental stages and adult tissues, with particularly high levels in vigorous cell proliferation (e.g., up to 318 TPM in multicell stage and up to 202 TPM in gonad tissue). Notably, PyMCM6 exhibited consistently high expression across development and across tissues (> 44 TPM), suggesting a key regulatory role in both development and tissue maintenance. Under low-pH stress, the expressions of PyMCMs were downregulated to varying degrees, with PyMCM5 showing the most significant reduction (|log2FC| up to 3.5), while PyMCM8 and PyMCM9 remained relatively stable. This pattern suggests a strategic response that scallops reduce DNA replication capacity (mediated by PyMCM2–7) but potentially maintain DNA repair functions (associated with PyMCM8/9 stability) to mitigate damage induced by low-pH, potentially explaining the intrinsic inhibition of cell proliferation. Quantitative real-time PCR and in situ hybridization further confirmed that low-pH stress inhibits PyMCMs expressions (p < 0.05), with the effect amplified as pH decreases. Collectively, these findings enhance our understanding of PyMCMs in regulating bivalve growth retardation under low-pH stress and provide valuable insights into the mechanisms of environmental adaptation in bivalves.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.10
自引率
3.30%
发文量
69
审稿时长
33 days
期刊介绍:
Comparative Biochemistry & Physiology (CBP) publishes papers in comparative, environmental and evolutionary physiology.
Part D: Genomics and Proteomics (CBPD), focuses on “omics” approaches to physiology, including comparative and functional genomics, metagenomics, transcriptomics, proteomics, metabolomics, and lipidomics. Most studies employ “omics” and/or system biology to test specific hypotheses about molecular and biochemical mechanisms underlying physiological responses to the environment. We encourage papers that address fundamental questions in comparative physiology and biochemistry rather than studies with a focus that is purely technical, methodological or descriptive in nature.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


