preussin衍生物的合成及其降脂活性。
IF 3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
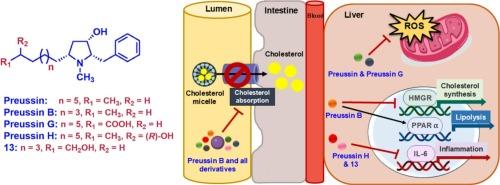
从l -苯丙氨酸开始,合成了11种天然和非天然的吡咯烷醇生物碱preussin类似物。我们的工作重点是通过关键的Sakurai烯丙化反应和交叉复分解反应构建具有功能化烷基侧链的全顺式吡咯烷醇-3-醇的有效策略,从而首次合成了其他六种前体蛋白酶类似物,包括四种天然产物,前体蛋白酶F, G, H和i。利用荧光胆固醇转运试验评估了11种合成类似物对人肠道Caco-2细胞胆固醇吸收的抑制作用。在统计学上,5 μg/mL的所有类似物减少胆固醇吸收,与40 μg/mL的阳性药物依折替米相当,对Caco-2细胞没有细胞毒性作用。合成preussin B的效价最高,为18.6%。通过实时荧光定量PCR检测肝脏脂质代谢相关基因和促炎因子的表达,进一步评价合成的类似物对人肝癌HepG2细胞的降脂作用。preussin B通过下调HMGR和上调PPARα显著调节肝脏脂质代谢基因,使preussin B成为一种新的有前景的降血脂药物。然而,在Caco-2和HepG2细胞系中,亲本化合物preussin在h2o2诱导的氧化应激条件下,通过显著清除细胞内活性氧,表现出最有效的抗氧化作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Synthesis and lipid-lowering activities of preussin derivatives
Syntheses of eleven natural and unnatural analogues of preussin, a pyrrolidin-3-ol alkaloid, have been achieved starting from L-phenylalanine. Our work highlighted an efficient strategy to construct the all cis pyrrolidin-3-ols bearing functionalized alkyl side chain via the key Sakurai allylation and cross metathesis reaction which led to the first syntheses of six other preussin analogues including the four natural products, preussins F, G, H and I. The eleven synthetic analogues were evaluated for their inhibitory effects on cholesterol absorption using fluorescent-cholesterol transport assay in human intestinal Caco-2 cells. All analogues at 5 μg/mL statistically reduced cholesterol absorption comparable to 40 μg/mL of a positive drug, ezetimibe, without cytotoxic effect to the Caco-2 cells. Synthetic preussin B exhibited the highest potency of 18.6 % reduction. The synthetic analogues were further evaluated for lipid-lowering effects in human hepatocellular carcinoma HepG2 cells via the expression of five genes related to hepatic lipid metabolism and pro-inflammatory cytokines using real-time PCR. Remarkably, preussin B significantly modulated hepatic lipid metabolism genes by down-regulating HMGR and up-regulating PPARα, rendering preussin B a new and promising candidate for hypolipidemic and hepatic lipid-lowering agent. Nevertheless, the parent compound, preussin, exhibited the most potent antioxidative effect by markedly scavenging intracellular reactive oxygen species in H2O2-induced oxidative stress condition in both Caco-2 and HepG2 cell lines.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


