运动、一氧化氮和代谢的交叉:揭示eNOS在骨骼肌及其他部位的作用。
IF 11.9
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
运动可以预防多种疾病,包括心脏代谢紊乱。然而,驱动这些适应的分子机制仍然不完全确定。内皮型一氧化氮合酶(eNOS)是一氧化氮(NO)的重要来源,参与调节运动后的葡萄糖摄取、脂肪酸代谢和线粒体重塑。eNOS在内皮细胞和非内皮细胞中均有表达,其对代谢的影响是多方面的。值得注意的是,eNOS在内皮细胞中高度表达,内皮细胞普遍存在于所有器官系统中,使它们能够与周围的细胞类型紧密结合。内皮的这一独特特征使eNOS能够影响局部微环境和跨器官系统的系统信号。本文综述了enos衍生NO在运动代谢中的作用。有证据表明,eNOS有助于改善代谢灵活性,增强线粒体功能和组织串扰。然而,不同实验模型的数据仍然是混杂的,既有支持的结果,也有相互矛盾的结果。总的来说,文献表明eNOS在促进运动诱导的代谢益处方面发挥着核心作用,尽管这依赖于环境。确定eNOS活性的具体机制和组织贡献仍然是未来研究的重要领域,与代谢性疾病的预防和治疗具有潜在的相关性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
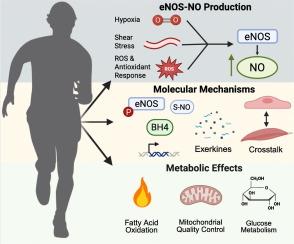
The intersection of exercise, nitric oxide, and metabolism: Unraveling the role of eNOS in skeletal muscle and beyond
Exercise protects against several diseases including cardiometabolic disorders. However, the molecular mechanisms driving these adaptations remain incompletely defined. Endothelial nitric oxide synthase (eNOS), a key source of nitric oxide (NO), is implicated in regulating glucose uptake, fatty acid metabolism, and mitochondrial remodeling in response to exercise. eNOS is expressed in both endothelial and non-endothelial cells and its effects on metabolism are multifaceted. Notably, eNOS is highly expressed in endothelial cells which are ubiquitous throughout all organ systems allowing them to closely integrate with surrounding cell types. This unique feature of the endothelium enables eNOS to influence both local microenvironments and signaling across organ systems. This review summarizes current findings on the role of eNOS-derived NO in exercise metabolism. Evidence suggests eNOS contributes to improved metabolic flexibility, enhanced mitochondrial function, and tissue crosstalk. However, data across experimental models remain mixed, with both supportive and conflicting results. Collectively, the literature indicates that eNOS plays a central, though context-dependent, role in facilitating exercise-induced metabolic benefits. Identifying the specific mechanisms and tissue contributions of eNOS activity remains an important area for future investigation, with potential relevance to metabolic disease prevention and treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Metabolism: clinical and experimental
医学-内分泌学与代谢
CiteScore
18.90
自引率
3.10%
发文量
310
审稿时长
16 days
期刊介绍:
Metabolism upholds research excellence by disseminating high-quality original research, reviews, editorials, and commentaries covering all facets of human metabolism.
Consideration for publication in Metabolism extends to studies in humans, animal, and cellular models, with a particular emphasis on work demonstrating strong translational potential.
The journal addresses a range of topics, including:
- Energy Expenditure and Obesity
- Metabolic Syndrome, Prediabetes, and Diabetes
- Nutrition, Exercise, and the Environment
- Genetics and Genomics, Proteomics, and Metabolomics
- Carbohydrate, Lipid, and Protein Metabolism
- Endocrinology and Hypertension
- Mineral and Bone Metabolism
- Cardiovascular Diseases and Malignancies
- Inflammation in metabolism and immunometabolism
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


