肉桂酸甲酯与2-羟丙基-β-环糊精的多方法结合与溶出
IF 5.2
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
本研究旨在利用探针超声和静电纺丝分别制备肉桂酸甲酯(MC)与2-羟丙基-β-环糊精(hp -β cd)包合物(IC)和纳米纤维(NF)网,并对其进行综合表征,以提高其生物利用度和热稳定性。紫外可见吸收光谱和荧光光谱分析表明,水溶液中MC与HPβCD分子间存在1:1的相互作用,表观缔合常数分别为275±10和505±8 M−1。场发射扫描电镜显示MC-HPβCD-NF无珠状纤维结构,平均纤维直径345±90 nm, MC-HPβCD-IC呈片状。粉末x射线衍射分析表明,在IC和NF中,MC的晶相完全转变为非晶结构,而不是物理混合物。热分析表明,IC和NF形式的MC稳定性显著增强,分解温度分别从145.5°C上升到349.7°C和366.7°C。傅里叶变换红外和核磁共振波谱的结构表征表明,IC和NF发生了明显的变化,HPβCD的空腔质子与MC的芳香质子之间存在明显的空间相关性。理论计算进一步阐明了MC在HPβCD空腔内的相互作用位点。MC在IC和NF中的溶解度都比其在纯水中的溶解度高约10倍。这些发现表明,用hpβ - cd包封和静电纺丝都是有效的策略,特别是后者,可以改善水溶性和热稳定性,使其在食品和芳香工业中有很大的应用前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
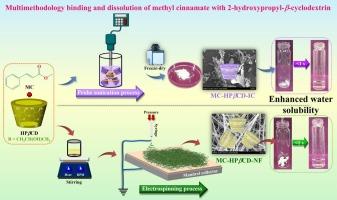
Deciphering the multimethodology binding and dissolution of methyl cinnamate with 2-hydroxypropyl-β-cyclodextrin
The present investigation aimed to fabricate and comprehensively characterize the inclusion complex (IC) and nanofibrous (NF) web of methyl cinnamate (MC) with 2-hydroxypropyl-β-cyclodextrin (HPβCD) using probe sonication and electrospinning, respectively, to enhance its bioavailability and thermal stability. UV–Vis absorption and fluorescence spectroscopy demonstrated 1:1 molecular interactions between MC and HPβCD in aqueous solution, with apparent association constants of 275 ± 10 and 505 ± 8 M−1. Field emission scanning electron microscopy revealed bead-free NF structures for MC-HPβCD-NF, with an average fiber diameter of 345 ± 90 nm, while MC-HPβCD-IC exhibited sheet-like flake morphologies. Powder X-ray diffraction analysis indicated the complete transformation of MC's crystalline phase into an amorphous structure in both IC and NF, in contrast to its physical mixture. Thermal analysis showed that MC in both IC and NF forms exhibited significantly enhanced stability, with decomposition temperatures increasing from 145.5 °C to 349.7 and 366.7 °C, respectively. Structural characterization by Fourier-transform infrared and nuclear magnetic resonance spectroscopy demonstrated distinct changes in both the IC and NF, with clear spatial correlations between the cavity protons of HPβCD and the aromatic protons of MC. Theoretical calculations further elucidated the interaction site of MC within the HPβCD cavity. The water solubility of MC in both IC and NF was approximately tenfold higher than its solubility in pure water. These findings suggest that both encapsulation and electrospinning of MC with HPβCD are effective strategies—particularly the latter—for improving aqueous solubility and thermal stability, making them promising for applications in the food and aroma industries.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Liquids
化学-物理:原子、分子和化学物理
CiteScore
10.30
自引率
16.70%
发文量
2597
审稿时长
78 days
期刊介绍:
The journal includes papers in the following areas:
– Simple organic liquids and mixtures
– Ionic liquids
– Surfactant solutions (including micelles and vesicles) and liquid interfaces
– Colloidal solutions and nanoparticles
– Thermotropic and lyotropic liquid crystals
– Ferrofluids
– Water, aqueous solutions and other hydrogen-bonded liquids
– Lubricants, polymer solutions and melts
– Molten metals and salts
– Phase transitions and critical phenomena in liquids and confined fluids
– Self assembly in complex liquids.– Biomolecules in solution
The emphasis is on the molecular (or microscopic) understanding of particular liquids or liquid systems, especially concerning structure, dynamics and intermolecular forces. The experimental techniques used may include:
– Conventional spectroscopy (mid-IR and far-IR, Raman, NMR, etc.)
– Non-linear optics and time resolved spectroscopy (psec, fsec, asec, ISRS, etc.)
– Light scattering (Rayleigh, Brillouin, PCS, etc.)
– Dielectric relaxation
– X-ray and neutron scattering and diffraction.
Experimental studies, computer simulations (MD or MC) and analytical theory will be considered for publication; papers just reporting experimental results that do not contribute to the understanding of the fundamentals of molecular and ionic liquids will not be accepted. Only papers of a non-routine nature and advancing the field will be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


